FANCM: Difference between revisions
| Line 27: | Line 27: | ||
==Disease linkage== |
==Disease linkage== |
||
Bi-allelic mutations in the FANCM gene were originally associated with [[Fanconi anemia]], although several individuals with FANCM deficiency do not appear to have the disorder.<ref name="pmid12724401">{{cite journal | vauthors = Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, Hoatlin ME, Wang W | display-authors = 6 | title = A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome | journal = Molecular and Cellular Biology | volume = 23 | issue = 10 | pages = 3417–26 | date = May 2003 | pmid = 12724401 | pmc = 164758 | doi = 10.1128/MCB.23.10.3417-3426.2003 }}</ref><ref>{{cite journal | vauthors = Bogliolo M, Bluteau D, Lespinasse J, Pujol R, Vasquez N, d'Enghien CD, Stoppa-Lyonnet D, Leblanc T, Soulier J, Surrallés J | display-authors = 6 | title = Biallelic truncating FANCM mutations cause early-onset cancer but not Fanconi anemia | journal = Genetics in Medicine | volume = 20 | issue = 4 | pages = 458–463 | date = April 2018 | pmid = 28837157 | doi = 10.1038/gim.2017.124 }}</ref><ref>{{cite journal | vauthors = Catucci I, Osorio A, Arver B, Neidhardt G, Bogliolo M, Zanardi F, Riboni M, Minardi S, Pujol R, Azzollini J, Peissel B, Manoukian S, De Vecchi G, Casola S, Hauke J, Richters L, Rhiem K, Schmutzler RK, Wallander K, Törngren T, Borg Å, Radice P, Surrallés J, Hahnen E, Ehrencrona H, Kvist A, Benitez J, Peterlongo P | display-authors = 6 | title = Individuals with FANCM biallelic mutations do not develop Fanconi anemia, but show risk for breast cancer, chemotherapy toxicity and may display chromosome fragility | journal = Genetics in Medicine | volume = 20 | issue = 4 | pages = 452–457 | date = April 2018 | pmid = 28837162 | doi = 10.1038/gim.2017.123 | url = https://ddd.uab.cat/pub/artpub/2018/182643/genmed_a2018v20p452.pdf }}</ref> Mono-allelic FANCM mutations were associated with breast cancer risk and especially with risk of developing ER-negative and TNBC disease subtypes.<ref name="PMID26130695">{{cite journal | vauthors = Peterlongo P, Catucci I, Colombo M, Caleca L, Mucaki E, Bogliolo M, Marin M, Damiola F, Bernard L, Pensotti V, Volorio S, Dall'Olio V, Meindl A, Bartram C, Sutter C, Surowy H, Sornin V, Dondon MG, Eon-Marchais S, Stoppa-Lyonnet D, Andrieu N, Sinilnikova OM, GENESIS, Mitchell G, James PA, Thompson E, kConFab, SWE-BRCA, Marchetti M, Verzeroli C, Tartari C, Capone GL, Putignano AL, Genuardi M, Medici V, Marchi I, Federico M, Tognazzo S, Matricardi L, Agata S, Dolcetti R, Della Puppa L, Cini G, Gismondi V, Viassolo V, Perfumo C, Mencarelli MA, Baldassarri M, Peissel B, Roversi G, Silvestri V, Rizzolo P, Spina F, Vivanet C, Tibiletti MG, Caligo MA, Gambino G, Tommasi S, Pilato B, Tondini C, Corna C, Bonanni B, Barile M, Osorio A, Benitez J, Balestrino L, Ottini L, Manoukian S, Pierotti MA, Renieri A, Varesco L, Couch FJ, Wang X, Devilee P, Hilbers FS, van Asperen CJ, Viel A, Montagna M, Cortesi L, Diez O, Balmaña J, Hauke J, Schmutzler RK, Papi L, Pujana MA, Lázaro C, Falanga A, Offit K, Vijai J, Campbell I, Burwinkel B, Kvist A, Ehrencrona H, Mazoyer S, Pizzamiglio S, Verderio P, Surralles J, Rogan PK, Radice P | display-authors = 6 | title = FANCM c.5791C>T nonsense mutation (rs144567652) induces exon skipping, affects DNA repair activity and is a familial breast cancer risk factor | journal = Human Molecular Genetics | volume = 24 | issue = 18 | pages = 5345–55 | date = September 2015 | pmid = 26130695 | pmc = 4550823 | doi = 10.1093/hmg/ddv251 }}</ref><ref name="PMID28033443">{{cite journal | vauthors = Neidhardt G, Hauke J, Ramser J, Groß E, Gehrig A, Müller CR, Kahlert AK, Hackmann K, Honisch E, Niederacher D, Heilmann-Heimbach S, Franke A, Lieb W, Thiele H, Altmüller J, Nürnberg P, Klaschik K, Ernst C, Ditsch N, Jessen F, Ramirez A, Wappenschmidt B, Engel C, Rhiem K, Meindl A, Schmutzler RK, Hahnen E|display-authors = 6 | title = Association Between Loss-of-Function Mutations Within the FANCM Gene and Early-Onset Familial Breast Cancer | journal = JAMA Oncology | volume = 3 | issue = 9 | pages = 1245–48 | date = September 2017 | pmid = 28033443 | pmc = 5824291 | doi = 10.1001/jamaoncol.2016.5592 }}</ref> A founder mutation in the Scandinavian population is also associated with a higher than average frequency of triple negative breast cancer in heterozygous carriers.<ref name="pmid25288723">{{cite journal | vauthors = Kiiski JI, Pelttari LM, Khan S, Freysteinsdottir ES, Reynisdottir I, Hart SN, Shimelis H, Vilske S, Kallioniemi A, Schleutker J, Leminen A, Bützow R, Blomqvist C, Barkardottir RB, Couch FJ, Aittomäki K, Nevanlinna H | display-authors = 6 | title = Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 111 | issue = 42 | pages = 15172–7 | date = October 2014 | pmid = 25288723 | pmc = 4210278 | doi = 10.1073/pnas.1407909111 }}</ref> FANCM carriers also have elevated levels of Ovarian cancer and other solid tumours<ref>{{cite journal | vauthors = Dicks E, Song H, Ramus SJ, Oudenhove EV, Tyrer JP, Intermaggio MP, Kar S, Harrington P, Bowtell DD, Group AS, Cicek MS, Cunningham JM, Fridley BL, Alsop J, Jimenez-Linan M, Piskorz A, Goranova T, Kent E, Siddiqui N, Paul J, Crawford R, Poblete S, Lele S, Sucheston-Campbell L, Moysich KB, Sieh W, McGuire V, Lester J, Odunsi K, Whittemore AS, Bogdanova N, Dürst M, Hillemanns P, Karlan BY, Gentry-Maharaj A, Menon U, Tischkowitz M, Levine D, Brenton JD, Dörk T, Goode EL, Gayther SA, Pharoah DP | display-authors = 6 | title = FANCM as a likely high grade serous ovarian cancer susceptibility gene | journal = Oncotarget | volume = 8 | issue = 31 | pages = 50930–50940 | date = August 2017 | pmid = 28881617 | pmc = 5584218 | doi = 10.18632/oncotarget.15871 }}</ref> |
Bi-allelic mutations in the FANCM gene were originally associated with [[Fanconi anemia]], although several individuals with FANCM deficiency do not appear to have the disorder.<ref name="pmid12724401">{{cite journal | vauthors = Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, Hoatlin ME, Wang W | display-authors = 6 | title = A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome | journal = Molecular and Cellular Biology | volume = 23 | issue = 10 | pages = 3417–26 | date = May 2003 | pmid = 12724401 | pmc = 164758 | doi = 10.1128/MCB.23.10.3417-3426.2003 }}</ref><ref>{{cite journal | vauthors = Bogliolo M, Bluteau D, Lespinasse J, Pujol R, Vasquez N, d'Enghien CD, Stoppa-Lyonnet D, Leblanc T, Soulier J, Surrallés J | display-authors = 6 | title = Biallelic truncating FANCM mutations cause early-onset cancer but not Fanconi anemia | journal = Genetics in Medicine | volume = 20 | issue = 4 | pages = 458–463 | date = April 2018 | pmid = 28837157 | doi = 10.1038/gim.2017.124 }}</ref><ref>{{cite journal | vauthors = Catucci I, Osorio A, Arver B, Neidhardt G, Bogliolo M, Zanardi F, Riboni M, Minardi S, Pujol R, Azzollini J, Peissel B, Manoukian S, De Vecchi G, Casola S, Hauke J, Richters L, Rhiem K, Schmutzler RK, Wallander K, Törngren T, Borg Å, Radice P, Surrallés J, Hahnen E, Ehrencrona H, Kvist A, Benitez J, Peterlongo P | display-authors = 6 | title = Individuals with FANCM biallelic mutations do not develop Fanconi anemia, but show risk for breast cancer, chemotherapy toxicity and may display chromosome fragility | journal = Genetics in Medicine | volume = 20 | issue = 4 | pages = 452–457 | date = April 2018 | pmid = 28837162 | doi = 10.1038/gim.2017.123 | url = https://ddd.uab.cat/pub/artpub/2018/182643/genmed_a2018v20p452.pdf }}</ref> Mono-allelic FANCM mutations were associated with breast cancer risk and especially with risk of developing ER-negative and TNBC disease subtypes.<ref name="PMID26130695">{{cite journal | vauthors = Peterlongo P, Catucci I, Colombo M, Caleca L, Mucaki E, Bogliolo M, Marin M, Damiola F, Bernard L, Pensotti V, Volorio S, Dall'Olio V, Meindl A, Bartram C, Sutter C, Surowy H, Sornin V, Dondon MG, Eon-Marchais S, Stoppa-Lyonnet D, Andrieu N, Sinilnikova OM, GENESIS, Mitchell G, James PA, Thompson E, kConFab, SWE-BRCA, Marchetti M, Verzeroli C, Tartari C, Capone GL, Putignano AL, Genuardi M, Medici V, Marchi I, Federico M, Tognazzo S, Matricardi L, Agata S, Dolcetti R, Della Puppa L, Cini G, Gismondi V, Viassolo V, Perfumo C, Mencarelli MA, Baldassarri M, Peissel B, Roversi G, Silvestri V, Rizzolo P, Spina F, Vivanet C, Tibiletti MG, Caligo MA, Gambino G, Tommasi S, Pilato B, Tondini C, Corna C, Bonanni B, Barile M, Osorio A, Benitez J, Balestrino L, Ottini L, Manoukian S, Pierotti MA, Renieri A, Varesco L, Couch FJ, Wang X, Devilee P, Hilbers FS, van Asperen CJ, Viel A, Montagna M, Cortesi L, Diez O, Balmaña J, Hauke J, Schmutzler RK, Papi L, Pujana MA, Lázaro C, Falanga A, Offit K, Vijai J, Campbell I, Burwinkel B, Kvist A, Ehrencrona H, Mazoyer S, Pizzamiglio S, Verderio P, Surralles J, Rogan PK, Radice P | display-authors = 6 | title = FANCM c.5791C>T nonsense mutation (rs144567652) induces exon skipping, affects DNA repair activity and is a familial breast cancer risk factor | journal = Human Molecular Genetics | volume = 24 | issue = 18 | pages = 5345–55 | date = September 2015 | pmid = 26130695 | pmc = 4550823 | doi = 10.1093/hmg/ddv251 }}</ref><ref name="PMID28033443">{{cite journal | vauthors = Neidhardt G, Hauke J, Ramser J, Groß E, Gehrig A, Müller CR, Kahlert AK, Hackmann K, Honisch E, Niederacher D, Heilmann-Heimbach S, Franke A, Lieb W, Thiele H, Altmüller J, Nürnberg P, Klaschik K, Ernst C, Ditsch N, Jessen F, Ramirez A, Wappenschmidt B, Engel C, Rhiem K, Meindl A, Schmutzler RK, Hahnen E|display-authors = 6 | title = Association Between Loss-of-Function Mutations Within the FANCM Gene and Early-Onset Familial Breast Cancer | journal = JAMA Oncology | volume = 3 | issue = 9 | pages = 1245–48 | date = September 2017 | pmid = 28033443 | pmc = 5824291 | doi = 10.1001/jamaoncol.2016.5592 }}</ref><ref name="PMID31700994">{{cite journal | vauthors = Figlioli G, Bogliolo M, Catucci I, Caleca L, Lasheras SV, Pujol R, Kiiski JI, Muranen TA, Barnes DR, Dennis J, Michailidou K, Bolla MK, Leslie G, Aalfs CM, ABCTB Investigators, Adank MA, Adlard J, Agata S, Cadoo K, Agnarsson BA, Ahearn T, Aittomäki K, Ambrosone CB, Andrews L, Anton-Culver H, Antonenkova NN, Arndt V, Arnold N, Aronson KJ, Arun BK, Asseryanis E, Auber B, Auvinen P, Azzollini J, Balmaña J, Barkardottir RB, Barrowdale D, Barwell J, Beane Freeman LE, Beauparlant CJ, Beckmann MW, Behrens S, Benitez J, Berger R, Bermisheva M, Blanco AM, Blomqvist C, Bogdanova NV, Bojesen A, Bojesen SE, Bonanni B, Borg A, Brady AF, Brauch H, Brenner H, Brüning T, Burwinkel B, Buys SS, Caldés T, Caliebe A, Caligo MA, Campa D, Campbell IG, Canzian F, Castelao JE, Chang-Claude J, Chanock SJ, Claes KBM, Clarke CL, Collavoli A, Conner TA, Cox DG, Cybulski C, Czene K, Daly MB, de la Hoya M, Devilee P, Diez O, Ding YC, Dite GS, Ditsch N, Domchek SM, Dorfling CM, Dos-Santos-Silva I, Durda K, Dwek M, Eccles DM, Ekici AB, Eliassen AH, Ellberg C, Eriksson M, Evans DG, Fasching PA, Figueroa J, Flyger H, Foulkes WD, Friebel TM, Friedman E, Gabrielson M, Gaddam P, Gago-Dominguez M, Gao C, Gapstur SM, Garber J, García-Closas M, García-Sáenz JA, Gaudet MM, Gayther SA, GEMO Study Collaborators, Giles GG, Glendon G, Godwin AK, Goldberg MS, Goldgar DE, Guénel P, Gutierrez-Barrera AM, Haeberle L, Haiman CA, Håkansson N, Hall P, Hamann U, Harrington PA, Hein A, Heyworth J, Hillemanns P, Hollestelle A, Hopper JL, Hosgood HD 3rd, Howell A, Hu C, Hulick PJ, Hunter DJ, Imyanitov EN, KConFab, Isaacs C, Jakimovska M, Jakubowska A, James P, Janavicius R, Janni W, John EM, Jones ME, Jung A, Kaaks R, Karlan BY, Khusnutdinova E, Kitahara CM, Konstantopoulou I, Koutros S, Kraft P, Lambrechts D, Lazaro C, Le Marchand L, Lester J, Lesueur F, Lilyquist J, Loud JT, Lu KH, Luben RN, Lubinski J, Mannermaa A, Manoochehri M, Manoukian S, Margolin S, Martens JWM, Maurer T, Mavroudis D, Mebirouk N, Meindl A, Menon U, Miller A, Montagna M, Nathanson KL, Neuhausen SL, Newman WG, Nguyen-Dumont T, Nielsen FC, Nielsen S, Nikitina-Zake L, Offit K, Olah E, Olopade OI, Olshan AF, Olson JE, Olsson H, Osorio A, Ottini L, Peissel B, Peixoto A, Peto J, Plaseska-Karanfilska D, Pocza T, Presneau N, Pujana MA, Punie K, Rack B, Rantala J, Rashid MU, Rau-Murthy R, Rennert G, Lejbkowicz F, Rhenius V, Romero A, Rookus MA, Ross EA, Rossing M, Rudaitis V, Ruebner M, Saloustros E, Sanden K, Santamariña M, Scheuner MT, Schmutzler RK, Schneider M, Scott C, Senter L, Shah M, Sharma P, Shu XO, Simard J, Singer CF, Sohn C, Soucy P, Southey MC, Spinelli JJ, Steele L, Stoppa-Lyonnet D, Tapper WJ, Teixeira MR, Terry MB, Thomassen M, Thompson J, Thull DL, Tischkowitz M, Tollenaar RAEM, Torres D, Troester MA, Truong T, Tung N, Untch M, Vachon CM, van Rensburg EJ, van Veen EM, Vega A, Viel A, Wappenschmidt B, Weitzel JN, Wendt C, Wieme G, Wolk A, Yang XR, Zheng W, Ziogas A, Zorn KK, Dunning AM, Lush M, Wang Q, McGuffog L, Parsons MT, Pharoah PDP, Fostira F, Toland AE, Andrulis IL, Ramus SJ, Swerdlow AJ, Greene MH, Chung WK, Milne RL, Chenevix-Trench G, Dörk T, Schmidt MK, Easton DF, Radice P, Hahnen E, Antoniou AC, Couch FJ, Nevanlinna H, Surrallés J, Peterlongo P|display-authors = 6 | title = The FANCM:p.Arg658* truncating variant is associated with risk of triple-negative breast cancer | journal = NPJ Breast Cancer | volume = 5 | issue = 38 | date = November 2019 | pmid = 31700994 | pmc = 6825205 | doi = 10.1038/s41523-019-0127-5 }}</ref> A founder mutation in the Scandinavian population is also associated with a higher than average frequency of triple negative breast cancer in heterozygous carriers.<ref name="pmid25288723">{{cite journal | vauthors = Kiiski JI, Pelttari LM, Khan S, Freysteinsdottir ES, Reynisdottir I, Hart SN, Shimelis H, Vilske S, Kallioniemi A, Schleutker J, Leminen A, Bützow R, Blomqvist C, Barkardottir RB, Couch FJ, Aittomäki K, Nevanlinna H | display-authors = 6 | title = Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 111 | issue = 42 | pages = 15172–7 | date = October 2014 | pmid = 25288723 | pmc = 4210278 | doi = 10.1073/pnas.1407909111 }}</ref> FANCM carriers also have elevated levels of Ovarian cancer and other solid tumours<ref>{{cite journal | vauthors = Dicks E, Song H, Ramus SJ, Oudenhove EV, Tyrer JP, Intermaggio MP, Kar S, Harrington P, Bowtell DD, Group AS, Cicek MS, Cunningham JM, Fridley BL, Alsop J, Jimenez-Linan M, Piskorz A, Goranova T, Kent E, Siddiqui N, Paul J, Crawford R, Poblete S, Lele S, Sucheston-Campbell L, Moysich KB, Sieh W, McGuire V, Lester J, Odunsi K, Whittemore AS, Bogdanova N, Dürst M, Hillemanns P, Karlan BY, Gentry-Maharaj A, Menon U, Tischkowitz M, Levine D, Brenton JD, Dörk T, Goode EL, Gayther SA, Pharoah DP | display-authors = 6 | title = FANCM as a likely high grade serous ovarian cancer susceptibility gene | journal = Oncotarget | volume = 8 | issue = 31 | pages = 50930–50940 | date = August 2017 | pmid = 28881617 | pmc = 5584218 | doi = 10.18632/oncotarget.15871 }}</ref> |
||
==FANCM as a therapeutic target in ALT cancer== |
==FANCM as a therapeutic target in ALT cancer== |
||
Revision as of 12:31, 26 February 2021
| Fanconi anemia, complementation group M | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FANCM | ||||||
| Alt. symbols | KIAA1596 | ||||||
| NCBI gene | 57697 | ||||||
| HGNC | 23168 | ||||||
| OMIM | 609644 | ||||||
| PDB | 4BXO | ||||||
| RefSeq | XM_048128 | ||||||
| UniProt | Q8IYD8 | ||||||
| Other data | |||||||
| EC number | 3.6.1.- | ||||||
| Locus | Chr. 14 q21.3 | ||||||
| |||||||
Fanconi anemia, complementation group M, also known as FANCM is a human gene.[1][2] It is an emerging target in cancer therapy, in particular cancers with specific genetic deficiencies.[3][4]
Function
The protein encoded by this gene, FANCM displays DNA binding against fork structures[5] and an ATPase activity associated with DNA branch migration. It is believed that FANCM in conjunction with other Fanconi anemia- proteins repair DNA at stalled replication forks, and stalled transcription structures called R-loops.[6][7]
The structure of the C-terminus of FANCM (amino acids 1799-2048), bound to a partner protein FAAP24, reveals how the protein complex recognises branched DNA.[5] A structure of amino acids 675-790 of FANCM reveal how the protein binds duplex DNA through a remodeling of the MHF1:MHF2 histone-like protein complex.

Disease linkage
Bi-allelic mutations in the FANCM gene were originally associated with Fanconi anemia, although several individuals with FANCM deficiency do not appear to have the disorder.[9][10][11] Mono-allelic FANCM mutations were associated with breast cancer risk and especially with risk of developing ER-negative and TNBC disease subtypes.[12][13][14] A founder mutation in the Scandinavian population is also associated with a higher than average frequency of triple negative breast cancer in heterozygous carriers.[15] FANCM carriers also have elevated levels of Ovarian cancer and other solid tumours[16]
FANCM as a therapeutic target in ALT cancer
Expression and activity of FANCM, is essential for the viability of cancers using Alternative Lengthening of Telomeres (ALT-associated cancers).[17][18][19] Several other synthetic lethal interactions have been observed for FANCM that may widen the targetability of the protein in therapeutic use.[17][4]
There are several potential ways in which FANCM activity could be targeted as an anti-cancer agent. In the context of ALT, one of the best targets may be a peptide domain of FANCM called MM2. Ectopic MM2 peptide (that acts as a dominant decoy) was sufficient to inhibit colony formation of ALT-associated cancer cells, but not telomerase-positive cancer cells.[18] This peptide works as a dominant interfering binder to RMI1:RMI2, and sequesters another DNA repair complex called the Bloom Syndrome complex away from FANCM.[7] As with FANCM depletion, this induces death through a “hyper-ALT” phenotype. An in vitro high-throughput screen for small molecule inhibitors of MM2-RMI1:2 interaction lead to the discovery of PIP-199.[20] This experimental drug also showed some discriminatory activity in killing of ALT-cells, compared to telomerase-positive cells.[18]
Meiosis
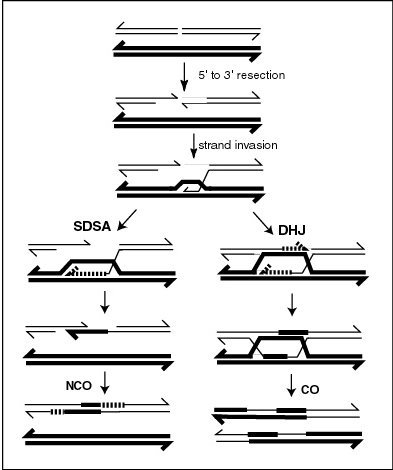
Recombination during meiosis is often initiated by a DNA double-strand break (DSB). During recombination, sections of DNA at the 5' ends of the break are cut away in a process called resection. In the strand invasion step that follows, an overhanging 3' end of the broken DNA molecule then "invades" the DNA of a homologous chromosome that is not broken forming a displacement loop (D-loop). After strand invasion, the further sequence of events may follow either of two main pathways leading to a crossover (CO) or a non-crossover (NCO) recombinant (see Genetic recombination and Homologous recombination). The pathway leading to a NCO is referred to as synthesis dependent strand annealing (SDSA).
In the plant Arabidopsis thaliana FANCM helicase antagonizes the formation of CO recombinants during meiosis, thus favoring NCO recombinants.[21] The FANCM helicase is required for genome stability in humans and yeast, and is a major factor limiting meiotic CO formation in A. thaliana.[22] A pathway involving another helicase, RECQ4A/B, also acts independently of FANCM to reduce CO recombination.[21] These two pathways likely act by unwinding different joint molecule substrates (e.g. nascent versus extended D-loops; see Figure).
Only about 4% of DSBs in A. thaliana are repaired by CO recombination;[22] the remaining 96% are likely repaired mainly by NCO recombination. Sequela-Arnaud et al.[21] suggested that CO numbers are restricted because of the long-term costs of CO recombination, that is, the breaking up of favorable genetic combinations of alleles built up by past natural selection.
In the fission yeast Schizosaccharomyces pombe, FANCM helicase also directs NCO recombination during meiosis.[23]
References
- ^ Nagase T, Kikuno R, Nakayama M, Hirosawa M, Ohara O (August 2000). "Prediction of the coding sequences of unidentified human genes. XVIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 7 (4): 273–81. doi:10.1093/dnares/7.4.271. PMID 10997877.
- ^ Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, et al. (September 2005). "A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M". Nature Genetics. 37 (9): 958–63. doi:10.1038/ng1626. PMC 2704909. PMID 16116422.
- ^ Pan X, Ahmed N, Kong J, Zhang D (2017-11-02). "Breaking the end: Target the replication stress response at the ALT telomeres for cancer therapy". Molecular & Cellular Oncology. 4 (6): e1360978. doi:10.1080/23723556.2017.1360978. PMC 5706943. PMID 29209649.
- ^ a b O'Rourke JJ, Bythell-Douglas R, Dunn EA, Deans AJ (October 2019). "ALT control, delete: FANCM as an anti-cancer target in Alternative Lengthening of Telomeres". Nucleus. 10 (1): 221–230. doi:10.1080/19491034.2019.1685246. PMC 6949022. PMID 31663812.
- ^ a b c Coulthard R, Deans AJ, Swuec P, Bowles M, Costa A, West SC, McDonald NQ (September 2013). "Architecture and DNA recognition elements of the Fanconi anemia FANCM-FAAP24 complex". Structure. 21 (9): 1648–58. doi:10.1016/j.str.2013.07.006. PMC 3763369. PMID 23932590.
- ^ Gari K, Décaillet C, Stasiak AZ, Stasiak A, Constantinou A (January 2008). "The Fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks". Molecular Cell. 29 (1): 141–8. doi:10.1016/j.molcel.2007.11.032. PMID 18206976.
- ^ a b Deans AJ, West SC (December 2009). "FANCM connects the genome instability disorders Bloom's Syndrome and Fanconi Anemia". Molecular Cell. 36 (6): 943–53. doi:10.1016/j.molcel.2009.12.006. PMID 20064461.
- ^ Walden H, Deans AJ (2014). "The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder". Annual Review of Biophysics. 43: 257–78. doi:10.1146/annurev-biophys-051013-022737. PMID 24773018.
- ^ Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, et al. (May 2003). "A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome". Molecular and Cellular Biology. 23 (10): 3417–26. doi:10.1128/MCB.23.10.3417-3426.2003. PMC 164758. PMID 12724401.
- ^ Bogliolo M, Bluteau D, Lespinasse J, Pujol R, Vasquez N, d'Enghien CD, et al. (April 2018). "Biallelic truncating FANCM mutations cause early-onset cancer but not Fanconi anemia". Genetics in Medicine. 20 (4): 458–463. doi:10.1038/gim.2017.124. PMID 28837157.
- ^ Catucci I, Osorio A, Arver B, Neidhardt G, Bogliolo M, Zanardi F, et al. (April 2018). "Individuals with FANCM biallelic mutations do not develop Fanconi anemia, but show risk for breast cancer, chemotherapy toxicity and may display chromosome fragility" (PDF). Genetics in Medicine. 20 (4): 452–457. doi:10.1038/gim.2017.123. PMID 28837162.
- ^ Peterlongo P, Catucci I, Colombo M, Caleca L, Mucaki E, Bogliolo M, et al. (September 2015). "FANCM c.5791C>T nonsense mutation (rs144567652) induces exon skipping, affects DNA repair activity and is a familial breast cancer risk factor". Human Molecular Genetics. 24 (18): 5345–55. doi:10.1093/hmg/ddv251. PMC 4550823. PMID 26130695.
- ^ Neidhardt G, Hauke J, Ramser J, Groß E, Gehrig A, Müller CR, et al. (September 2017). "Association Between Loss-of-Function Mutations Within the FANCM Gene and Early-Onset Familial Breast Cancer". JAMA Oncology. 3 (9): 1245–48. doi:10.1001/jamaoncol.2016.5592. PMC 5824291. PMID 28033443.
- ^ Figlioli G, Bogliolo M, Catucci I, Caleca L, Lasheras SV, Pujol R, et al. (November 2019). "The FANCM:p.Arg658* truncating variant is associated with risk of triple-negative breast cancer". NPJ Breast Cancer. 5 (38). doi:10.1038/s41523-019-0127-5. PMC 6825205. PMID 31700994.
{{cite journal}}: Vancouver style error: name in name 15 (help) - ^ Kiiski JI, Pelttari LM, Khan S, Freysteinsdottir ES, Reynisdottir I, Hart SN, et al. (October 2014). "Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer". Proceedings of the National Academy of Sciences of the United States of America. 111 (42): 15172–7. doi:10.1073/pnas.1407909111. PMC 4210278. PMID 25288723.
- ^ Dicks E, Song H, Ramus SJ, Oudenhove EV, Tyrer JP, Intermaggio MP, et al. (August 2017). "FANCM as a likely high grade serous ovarian cancer susceptibility gene". Oncotarget. 8 (31): 50930–50940. doi:10.18632/oncotarget.15871. PMC 5584218. PMID 28881617.
- ^ a b Pan X, Drosopoulos WC, Sethi L, Madireddy A, Schildkraut CL, Zhang D (July 2017). "FANCM, BRCA1, and BLM cooperatively resolve the replication stress at the ALT telomeres". Proceedings of the National Academy of Sciences of the United States of America. 114 (29): E5940–E5949. doi:10.1073/pnas.1708065114. PMC 5530707. PMID 28673972.
- ^ a b c Lu R, O'Rourke JJ, Sobinoff AP, Allen JA, Nelson CB, Tomlinson CG, et al. (May 2019). "The FANCM-BLM-TOP3A-RMI complex suppresses alternative lengthening of telomeres (ALT)". Nature Communications. 10 (1): 2252. doi:10.1038/s41467-019-10180-6. PMC 6538672. PMID 31138797.
- ^ Silva B, Pentz R, Figueira AM, Arora R, Lee YW, Hodson C, et al. (May 2019). "FANCM limits ALT activity by restricting telomeric replication stress induced by deregulated BLM and R-loops". Nature Communications. 10 (1): 2253. doi:10.1038/s41467-019-10179-z. PMC 6538666. PMID 31138795.
- ^ Voter AF, Manthei KA, Keck JL (July 2016). "A High-Throughput Screening Strategy to Identify Protein-Protein Interaction Inhibitors That Block the Fanconi Anemia DNA Repair Pathway". Journal of Biomolecular Screening. 21 (6): 626–33. doi:10.1177/1087057116635503. PMC 5038921. PMID 26962873.
- ^ a b c Séguéla-Arnaud M, Crismani W, Larchevêque C, Mazel J, Froger N, Choinard S, et al. (April 2015). "Multiple mechanisms limit meiotic crossovers: TOP3α and two BLM homologs antagonize crossovers in parallel to FANCM". Proceedings of the National Academy of Sciences of the United States of America. 112 (15): 4713–8. doi:10.1073/pnas.1423107112. PMC 4403193. PMID 25825745.
- ^ a b Crismani W, Girard C, Froger N, Pradillo M, Santos JL, Chelysheva L, et al. (June 2012). "FANCM limits meiotic crossovers". Science. 336 (6088): 1588–90. doi:10.1126/science.1220381. PMID 22723424. S2CID 14570996.
- ^ Lorenz A, Osman F, Sun W, Nandi S, Steinacher R, Whitby MC (June 2012). "The fission yeast FANCM ortholog directs non-crossover recombination during meiosis". Science. 336 (6088): 1585–8. doi:10.1126/science.1220111. PMC 3399777. PMID 22723423.
External links
- FANCM protein, human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
