Strobilurin: Difference between revisions
Add book reference with details of mode of action and much else |
Correct structure for strobilurin A with secondary references. Tidy up citations |
||
| Line 1: | Line 1: | ||
'''Strobilurins''' are a group of natural products and their synthetic analogs. A number of strobilurins are used in [[agriculture]] as [[fungicide]]s. They are part of the larger group of [[QoI|Q{{sub|o}}Is]] (Quinone outside Inhibitors), which act to inhibit the [[respiratory chain]] at the level of [[Complex III]]. |
'''Strobilurins''' are a group of natural products and their synthetic analogs. A number of strobilurins are used in [[agriculture]] as [[fungicide]]s. They are part of the larger group of [[QoI|Q{{sub|o}}Is]] (Quinone outside Inhibitors), which act to inhibit the [[respiratory chain]] at the level of [[Complex III]]. |
||
The first parent natural products, strobilurins A and B, were extracted from the fungus ''[[Strobilurus tenacellus]]''. |
The first parent natural products, strobilurins A and B, were extracted from the fungus ''[[Strobilurus tenacellus]]''.<ref>{{cite journal |doi=10.1002/cber.19781110806 |title=Antibiotika aus Basidiomyceten, III. Strobilurin a und B, antifungische Stoffwechselprodukte aus Strobilurus tenacellus |year=1978 |last1=Schramm |first1=Georg |last2=Steglich |first2=Wolfgang |last3=Anke |first3=Timm |last4=Oberwinkler |first4=Franz |journal=Chemische Berichte |volume=111 |issue=8 |pages=2779–2784 }}</ref> |
||
| ⚫ | Commercial strobilurin fungicides<ref>Peter Jeschke, Matthias Witschel, Wolfgang Krämer, Ulrich Schirmer (eds.): ''Modern Crop Protection Compounds'', 3rd edition, Wiley-VCH, 2019, {{ISBN|978-3-527-34089-7}}.</ref> were developed through optimization of photostability and activity.<ref>{{cite journal |doi=10.1002/(SICI)1521-3773(19990517)38:10<1328::AID-ANIE1328>3.0.CO;2-1 |title=Strobilurins: Evolution of a New Class of Active Substances |year=1999 |last1=Sauter |first1=Hubert |last2=Steglich |first2=Wolfgang |last3=Anke |first3=Timm |journal=Angewandte Chemie International Edition |volume=38 |issue=10 |pages=1328–1349 |pmid=29711574 }}</ref> |
||
<ref> |
|||
{{Cite journal |
|||
| title = Strobilurin A and B, antifungal metabolites from Strobilurus tenacellus |
|||
| pages = 8 |
|||
| author = Georg Schramm, Wolfgang Steglich, Timm Anke, Franz Oberwinkler |
|||
| doi = 10.1002/cber.19781110806 |
|||
| journal = Chem. Ber. |
|||
| volume = 111 |
|||
| year = 1978}} |
|||
</ref> |
|||
| ⚫ | |||
<ref> |
|||
{{Cite journal |
|||
| title = Strobilurins: Evolution of a new class of active substances. |
|||
| pages = 1328─1349 |
|||
| author = Hubert Sauter, Wolfgang Steglich, Timm Anke |
|||
| journal = Angew. Chem. Int. Ed. Engl. |
|||
| volume = 38 |
|||
| year = 1999}} |
|||
([https://doi.org/10.1002/(SICI)1521-3773(19990517)38:10%3C1328::AID-ANIE1328%3E3.0.CO;2-1 doi link]) |
|||
</ref> |
|||
Strobilurins represented a major development in fungus-based fungicides. First released in 1996, there are now ten major strobilurin fungicides on the market, which account for 23-25 % of the global fungicide sales.<ref> |
Strobilurins represented a major development in fungus-based fungicides. First released in 1996, there are now ten major strobilurin fungicides on the market, which account for 23-25 % of the global fungicide sales.<ref> |
||
{{Cite journal |
{{Cite journal |
||
| Line 38: | Line 18: | ||
==Natural strobilurins== |
==Natural strobilurins== |
||
===Strobilurin A=== |
===Strobilurin A=== |
||
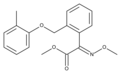
:[[Image:Strobilurin |
:[[Image:Strobilurin A.svg|200px]] |
||
Strobilurin A (also known as mucidin) is produced by ''[[Oudemansiella mucida]]'', ''[[Strobilurus tenacellus]]'', ''[[Bolinea lutea]]'', and others.<ref name=Anke>{{cite book |doi=10.1007/978-3-662-10378-4_5 |chapter=Non-β-Lactam Antibiotics |title=Industrial Applications |year=2002 |last1=Anke |first1=T. |last2=Erkel |first2=G. |pages=101–104 |isbn=978-3-642-07481-3 }}</ref><ref name="Zakharychev-Kovalenko-1998">{{cite journal | last=Zakharychev | first=Vladimir V | last2=Kovalenko | first2=Leonid V | title=Natural compounds of the strobilurin series and their synthetic analogues as cell respiration inhibitors | journal={{bracket|[[Russian Chemical Reviews]]}} (Успехи химии) | publisher=[[IOP Publishing]] | volume=67 | issue=6 | date=1998-06-30 | issn=0036-021X | doi=10.1070/rc1998v067n06abeh000426 | pages=535–544 | s2cid=95676421}}</ref> When first isolated it was incorrectly assigned as the E E E [[geometric isomer]] but was later identified by [[total synthesis]] as being the E Z E isomer, as shown.<ref name=Schaefer>{{cite book |doi=10.1007/978-3-642-54461-3_8 |chapter=Agrochemicals: 8.2 Strobilurins |title=Natural Products in the Chemical Industry |year=2014 |last1=Schaefer |first1=Bernd |pages=688–704 |isbn=978-3-642-54460-6 }}</ref>{{rp|694}} |
|||
===Strobilurin B=== |
===Strobilurin B=== |
||
:[[Image:Strobilurin-B-2D-skeletal.png|120px]] |
:[[Image:Strobilurin-B-2D-skeletal.png|120px]] |
||
Revision as of 15:22, 23 October 2022
Strobilurins are a group of natural products and their synthetic analogs. A number of strobilurins are used in agriculture as fungicides. They are part of the larger group of QoIs (Quinone outside Inhibitors), which act to inhibit the respiratory chain at the level of Complex III.
The first parent natural products, strobilurins A and B, were extracted from the fungus Strobilurus tenacellus.[1] Commercial strobilurin fungicides[2] were developed through optimization of photostability and activity.[3] Strobilurins represented a major development in fungus-based fungicides. First released in 1996, there are now ten major strobilurin fungicides on the market, which account for 23-25 % of the global fungicide sales.[4] Examples of commercialized strobilurin derivatives are azoxystrobin, kresoxim-methyl, picoxystrobin, fluoxastrobin, oryzastrobin, dimoxystrobin, pyraclostrobin and trifloxystrobin.
Strobilurins are mostly contact fungicides with a long half time as they are absorbed into the cuticle and not transported any further. They have a suppressive effect on other fungi, reducing competition for nutrients; they inhibit electron transfer in mitochondria, disrupting metabolism and preventing growth of the target fungi.[5]
Natural strobilurins
Strobilurin A
Strobilurin A (also known as mucidin) is produced by Oudemansiella mucida, Strobilurus tenacellus, Bolinea lutea, and others.[6][7] When first isolated it was incorrectly assigned as the E E E geometric isomer but was later identified by total synthesis as being the E Z E isomer, as shown.[8]: 694
Strobilurin B
Strobilurin B is produced by S. tenacellus.[7]
Strobilurin C
Strobilurin C is produced by X. longipes and X. melanotricha.[7]
Strobilurin D
Strobilurin D is produced by Cyphellopsis anomala.[7]
Strobilurin E
Strobilurin E is produced by Crepidotus fulvotomentosus.[7]
Strobilurin F
Strobilurin F is produced by C. anomala (F-1) and B. lutea (F-2).[7]
Strobilurin G
Strobilurin G is produced by B. lutea.[7]
Strobilurin H
Strobilurin H is produced by B. lutea.[7]
Strobilurin X
Strobilurin X is produced by O. mucida.[7]
Hydroxystrobilurin D
Hydroxystrobilurin D is produced by Mycena sanguinolenta.[7]
9-Methoxystrobilurin A
9-Methoxystrobilurin A is produced by Favolaschia spp.[7]
9-Methoxystrobilurin K
9-Methoxystrobilurin K is produced by Favolaschia spp.[7]
Oudemansin A
Oudemansin A is produced by O. mucida.[7]
Oudemansin B
Oudemansin B is produced by X. longipes and X. melanotricha.[7]
Oudemansin X
Oudemansin X is produced by O. radicata.[7]
Synthetic strobilurins
Azoxystrobin
See also
References
- ^ Schramm, Georg; Steglich, Wolfgang; Anke, Timm; Oberwinkler, Franz (1978). "Antibiotika aus Basidiomyceten, III. Strobilurin a und B, antifungische Stoffwechselprodukte aus Strobilurus tenacellus". Chemische Berichte. 111 (8): 2779–2784. doi:10.1002/cber.19781110806.
- ^ Peter Jeschke, Matthias Witschel, Wolfgang Krämer, Ulrich Schirmer (eds.): Modern Crop Protection Compounds, 3rd edition, Wiley-VCH, 2019, ISBN 978-3-527-34089-7.
- ^ Sauter, Hubert; Steglich, Wolfgang; Anke, Timm (1999). "Strobilurins: Evolution of a New Class of Active Substances". Angewandte Chemie International Edition. 38 (10): 1328–1349. doi:10.1002/(SICI)1521-3773(19990517)38:10<1328::AID-ANIE1328>3.0.CO;2-1. PMID 29711574.
- ^
Juliet D. Tang, Tina Ciaramitaro, Maria Tomaso-Peterson, Susan V. Diehl (2017). "Activity of Two Strobilurin Fungicides Against Three Species of Decay Fungi in Agar Plate Tests". Proc. IRG Annual Meeting: IRG/WP 17-30704.
{{cite journal}}: CS1 maint: multiple names: authors list (link) (pdf link) - ^ Schaefer, Bernd (2014). "Agrochemicals: 8.2 Strobilurins". Natural Products in the Chemical Industry. pp. 688–704. doi:10.1007/978-3-642-54461-3_8. ISBN 978-3-642-54460-6.
- ^ Anke, T.; Erkel, G. (2002). "Non-β-Lactam Antibiotics". Industrial Applications. pp. 101–104. doi:10.1007/978-3-662-10378-4_5. ISBN 978-3-642-07481-3.
- ^ a b c d e f g h i j k l m n o Zakharychev, Vladimir V; Kovalenko, Leonid V (1998-06-30). "Natural compounds of the strobilurin series and their synthetic analogues as cell respiration inhibitors". [Russian Chemical Reviews] (Успехи химии). 67 (6). IOP Publishing: 535–544. doi:10.1070/rc1998v067n06abeh000426. ISSN 0036-021X. S2CID 95676421.
- ^ Schaefer, Bernd (2014). "Agrochemicals: 8.2 Strobilurins". Natural Products in the Chemical Industry. pp. 688–704. doi:10.1007/978-3-642-54461-3_8. ISBN 978-3-642-54460-6.
External links
- Resistance to strobilurins in the southern USA, archived from the original on 2019-09-08, retrieved 2019-09-08
- Agricultural mycocides for the 21st century: strobilurins, archived from the original on 2019-09-08, retrieved 2019-09-08, from: David Moore, Geoffrey D. Robson, Anthony P. J. Trinci, 21st Century Guidebook to Fungi, 2nd edition.