Passerini reaction: Difference between revisions
Citation bot (talk | contribs) Alter: pages. Add: bibcode, pmc, pmid, doi, issue, authors 1-1. Removed parameters. Formatted dashes. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Abductive | Category:Name reactions | #UCB_Category 264/523 |
Lead edited. Copied from User:Rebekah Greenwood/Sandbox |
||
| Line 10: | Line 10: | ||
{{Use dmy dates|date=June 2013}} |
{{Use dmy dates|date=June 2013}} |
||
The '''Passerini reaction''' is a [[chemical reaction]] involving an [[isocyanide]], an [[aldehyde]] (or [[ketone]]), and a [[carboxylic acid]] to form a α-[[acyloxy]] [[amide]].<ref>Passerini, M.; Simone, L. ''Gazz. Chim. Ital.'' '''1921''', ''51'', 126–29.</ref><ref>Passerini, M.; Ragni, G. ''Gazz. Chim. Ital.'' '''1931''', ''61'', 964–69.</ref><ref>{{cite book|author1=Banfi, L. |author2=Riva, R. |journal=Org. React.|year=2005|volume=65|pages=1–140|doi=10.1002/0471264180.or065.01|title=The Passerini Reaction|isbn=978-0471264187}}.</ref><ref>{{Cite journal |last1=Kazemizadeh |first1=A.R. |last2=Ramazani |first2=A. |date=2012 |title=Synthetic applications of Passerini reaction |journal=Curr. Org. Chem. |volume=16 |issue=4 |pages=418–450|doi=10.2174/138527212799499868 }}</ref><ref>{{Cite journal |last1=Banfi |first1=L. |last2=Basso |first2=A. |last3=Lambruschini |first3=C. |last4=Moni |first4=L. |last5=Riva |first5=R. |date=2021 |title=The 100 facets of the Passerini reaction |url= |journal=Chem. Sci. |volume=12 |issue=47 |pages=15445–15472|doi=10.1039/D1SC03810A |pmid=35003575 |pmc=8654045 }}</ref> |
The '''Passerini reaction''' is a [[chemical reaction]] involving an [[isocyanide]], an [[aldehyde]] (or [[ketone]]), and a [[carboxylic acid]] to form a α-[[acyloxy]] [[amide]].<ref>Passerini, M.; Simone, L. ''Gazz. Chim. Ital.'' '''1921''', ''51'', 126–29.</ref><ref>Passerini, M.; Ragni, G. ''Gazz. Chim. Ital.'' '''1931''', ''61'', 964–69.</ref><ref>{{cite book|author1=Banfi, L. |author2=Riva, R. |journal=Org. React.|year=2005|volume=65|pages=1–140|doi=10.1002/0471264180.or065.01|title=The Passerini Reaction|isbn=978-0471264187}}.</ref><ref>{{Cite journal |last1=Kazemizadeh |first1=A.R. |last2=Ramazani |first2=A. |date=2012 |title=Synthetic applications of Passerini reaction |journal=Curr. Org. Chem. |volume=16 |issue=4 |pages=418–450|doi=10.2174/138527212799499868 }}</ref><ref>{{Cite journal |last1=Banfi |first1=L. |last2=Basso |first2=A. |last3=Lambruschini |first3=C. |last4=Moni |first4=L. |last5=Riva |first5=R. |date=2021 |title=The 100 facets of the Passerini reaction |url= |journal=Chem. Sci. |volume=12 |issue=47 |pages=15445–15472|doi=10.1039/D1SC03810A |pmid=35003575 |pmc=8654045 }}</ref> This addition reaction is one of the oldest [[isocyanide]]-based multicomponent reactions (IMCR) and was first described in 1921 by Mario Passerini in Florence, Italy.<ref name=":10">{{Cite journal |last=Tuten |first=Bryan T. |last2=Bui |first2=Aaron H. |last3=Wiedbrauk |first3=Sandra |last4=Truong |first4=Vinh X. |last5=Raston |first5=Colin L. |last6=Barner-Kowollik |first6=Christopher |date=2021-08-19 |title=Four component Passerini polymerization of bulky monomers under high shear flow |url=https://pubs.rsc.org/en/content/articlelanding/2021/cc/d1cc02984c |journal=Chemical Communications |language=en |volume=57 |issue=67 |pages=8328–8331 |doi=10.1039/D1CC02984C |issn=1364-548X}}</ref><ref name=":1">{{Cite journal |last1=Antenucci |first1=Achille |last2=Marra |first2=Francesco |last3=Dughera |first3=Stefano |date=2021 |title=Silica gel-immobilised chiral 1, 2-benzenedisulfonimide: a Brønsted acid heterogeneous catalyst for enantioselective multicomponent Passerini reaction |journal=RSC Advances |volume=11 |issue=42 |pages=26083–26092 |bibcode=2021RSCAd..1126083A |doi=10.1039/D1RA05297G |pmc=9037113 |pmid=35479468}}</ref> It is typically carried out in [[Polar aprotic solvent|aprotic]] solvents but can also be performed in ionic liquids such as water or [[Deep eutectic solvent|Deep Eutectic solvents]] (DESs).<ref name=":1" /> It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in [[Combinatorial chemistry|combinatorial]] and [[medicinal chemistry]] with recent utility in [[green chemistry]] and [[polymer chemistry]].<ref name=":10" /><ref name=":13">{{Cite journal |last=Abbasi |first=Elham |last2=Aval |first2=Sedigheh Fekri |last3=Akbarzadeh |first3=Abolfazl |last4=Milani |first4=Morteza |last5=Nasrabadi |first5=Hamid Tayefi |last6=Joo |first6=Sang Woo |last7=Hanifehpour |first7=Younes |last8=Nejati-Koshki |first8=Kazem |last9=Pashaei-Asl |first9=Roghiyeh |date=2014-05-21 |title=Dendrimers: synthesis, applications, and properties |url=https://doi.org/10.1186/1556-276X-9-247 |journal=Nanoscale Research Letters |volume=9 |issue=1 |pages=247 |doi=10.1186/1556-276X-9-247 |issn=1556-276X |pmc=PMC4074873 |pmid=24994950}}</ref><ref>Dömling, A.; Ugi, I. ''[[Angew. Chem. Int. Ed. Engl.]]'' '''2000''', ''39'', 3168–3210. (Review)</ref> As isocyanides exhibit high functional group tolerance, [[chemoselectivity]], [[regioselectivity]], and [[stereoselectivity]], the Passerini reaction has a wide range of synthetic applications. |
||
<ref name=":10" /><ref name=":0">''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 {{ISBN|0-471-68260-8}}</ref><ref name=":2">{{Cite journal |last1=Taran |first1=Jafar |last2=Ramazani |first2=Ali |last3=Joo |first3=Sang Woo |last4=Ślepokura |first4=Katarzyna |last5=Lis |first5=Tadeusz |date=2014 |title=Synthesis of Novel a-(Acyloxy)-a-(quinolin-4-yl)acetamides by a ThreeComponent Reaction between an Isocyanide, Quinoline-4-carbaldehyde, and Arenecarboxylic Acids |journal=Helvetica Chimica Acta |volume=97 |pages=1088–1096 |doi=10.1002/hlca.201300378}}</ref><ref name=":14">{{Cite journal |last=Wahby |first=Yasmin |last2=Abdel-Hamid |first2=Hamida |last3=Ayoup |first3=Mohammed Salah |date=2022 |title=Two decades of recent advances of Passerini reactions: synthetic and potential pharmaceutical applications |url=http://xlink.rsc.org/?DOI=D1NJ03832J |journal=New Journal of Chemistry |language=en |volume=46 |issue=4 |pages=1445–1468 |doi=10.1039/D1NJ03832J |issn=1144-0546}}</ref>[[File:Passerini reaction.svg|498px|center|The Passerini reaction]] |
|||
[[File:Passerini reaction.svg|498px|center|The Passerini reaction]] |
|||
This [[organic reaction]] was discovered by Mario Passerini in 1921 in [[Florence]], Italy. It is the first isocyanide based [[multi-component reaction]] developed, and currently plays a central role in [[combinatorial chemistry]].<ref>Dömling, A.; Ugi, I. ''[[Angew. Chem. Int. Ed. Engl.]]'' '''2000''', ''39'', 3168–3210. (Review)</ref> |
|||
Recently, Denmark ''et al.'' have developed an enantioselective [[catalyst]] for asymmetric Passerini reactions.<ref>Denmark, S. E.; Fan, Y. ''[[J. Org. Chem.]]'' '''2005''', ''70'', 9667–76. {{doi|10.1021/jo050549m}}</ref> |
Recently, Denmark ''et al.'' have developed an enantioselective [[catalyst]] for asymmetric Passerini reactions.<ref>Denmark, S. E.; Fan, Y. ''[[J. Org. Chem.]]'' '''2005''', ''70'', 9667–76. {{doi|10.1021/jo050549m}}</ref> |
||
Revision as of 21:00, 13 November 2022
| Passerini reaction | |
|---|---|
| Named after | Mario Passerini |
| Reaction type | Carbon-carbon bond forming reaction |
| Identifiers | |
| Organic Chemistry Portal | passerini-reaction |
| RSC ontology ID | RXNO:0000244 |
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde (or ketone), and a carboxylic acid to form a α-acyloxy amide.[1][2][3][4][5] This addition reaction is one of the oldest isocyanide-based multicomponent reactions (IMCR) and was first described in 1921 by Mario Passerini in Florence, Italy.[6][7] It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or Deep Eutectic solvents (DESs).[7] It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry.[6][8][9] As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.
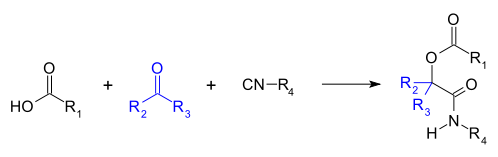
Recently, Denmark et al. have developed an enantioselective catalyst for asymmetric Passerini reactions.[13]
Reaction mechanism
Two reaction pathways have been hypothesized.
Ionic mechanism
In polar solvents such as methanol or water, the reaction proceeds by protonation of the carbonyl followed by nucleophilic addition of the isocyanide to give the nitrilium ion 3. Addition of a carboxylate gives intermediate 4. Acyl group transfer and amide tautomerization give the desired ester 5.[14][15]
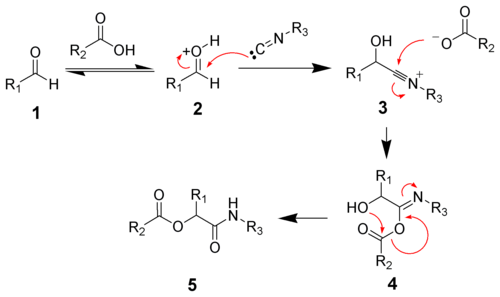
Concerted mechanism
In non-polar solvents and at high concentration a concerted mechanism is likely:[16]

This mechanism involves a trimolecular reaction between the isocyanide (R–NC), the carboxylic acid, and the carbonyl in a sequence of nucleophilic additions. The transition state TS# is depicted as a 5-membered ring with partial covalent or double bonding. The second step of the Passerini reaction is an acyl transfer to the neighboring hydroxyl group. There is support for this reaction mechanism: the reaction proceeds in relatively non-polar solvents (in line with transition state) and the reaction kinetics depend on all three reactants. This reaction is a good example of a convergent synthesis.
Scope
The Passerini reaction is used in many multicomponent reactions. For instance one preceded by a Horner-Wadsworth-Emmons reaction and forming a depsipeptide:[17]

Passerini multicomponent reactions have found use in the preparation of polymers from renewable materials.[18]
See also
References
- ^ Passerini, M.; Simone, L. Gazz. Chim. Ital. 1921, 51, 126–29.
- ^ Passerini, M.; Ragni, G. Gazz. Chim. Ital. 1931, 61, 964–69.
- ^ Banfi, L.; Riva, R. (2005). The Passerini Reaction. Vol. 65. pp. 1–140. doi:10.1002/0471264180.or065.01. ISBN 978-0471264187.
{{cite book}}:|journal=ignored (help). - ^ Kazemizadeh, A.R.; Ramazani, A. (2012). "Synthetic applications of Passerini reaction". Curr. Org. Chem. 16 (4): 418–450. doi:10.2174/138527212799499868.
- ^ Banfi, L.; Basso, A.; Lambruschini, C.; Moni, L.; Riva, R. (2021). "The 100 facets of the Passerini reaction". Chem. Sci. 12 (47): 15445–15472. doi:10.1039/D1SC03810A. PMC 8654045. PMID 35003575.
- ^ a b c Tuten, Bryan T.; Bui, Aaron H.; Wiedbrauk, Sandra; Truong, Vinh X.; Raston, Colin L.; Barner-Kowollik, Christopher (19 August 2021). "Four component Passerini polymerization of bulky monomers under high shear flow". Chemical Communications. 57 (67): 8328–8331. doi:10.1039/D1CC02984C. ISSN 1364-548X.
- ^ a b Antenucci, Achille; Marra, Francesco; Dughera, Stefano (2021). "Silica gel-immobilised chiral 1, 2-benzenedisulfonimide: a Brønsted acid heterogeneous catalyst for enantioselective multicomponent Passerini reaction". RSC Advances. 11 (42): 26083–26092. Bibcode:2021RSCAd..1126083A. doi:10.1039/D1RA05297G. PMC 9037113. PMID 35479468.
- ^ Abbasi, Elham; Aval, Sedigheh Fekri; Akbarzadeh, Abolfazl; Milani, Morteza; Nasrabadi, Hamid Tayefi; Joo, Sang Woo; Hanifehpour, Younes; Nejati-Koshki, Kazem; Pashaei-Asl, Roghiyeh (21 May 2014). "Dendrimers: synthesis, applications, and properties". Nanoscale Research Letters. 9 (1): 247. doi:10.1186/1556-276X-9-247. ISSN 1556-276X. PMC 4074873. PMID 24994950.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Dömling, A.; Ugi, I. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168–3210. (Review)
- ^ The Passirini Reaction L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 ISBN 0-471-68260-8
- ^ Taran, Jafar; Ramazani, Ali; Joo, Sang Woo; Ślepokura, Katarzyna; Lis, Tadeusz (2014). "Synthesis of Novel a-(Acyloxy)-a-(quinolin-4-yl)acetamides by a ThreeComponent Reaction between an Isocyanide, Quinoline-4-carbaldehyde, and Arenecarboxylic Acids". Helvetica Chimica Acta. 97: 1088–1096. doi:10.1002/hlca.201300378.
- ^ Wahby, Yasmin; Abdel-Hamid, Hamida; Ayoup, Mohammed Salah (2022). "Two decades of recent advances of Passerini reactions: synthetic and potential pharmaceutical applications". New Journal of Chemistry. 46 (4): 1445–1468. doi:10.1039/D1NJ03832J. ISSN 1144-0546.
- ^ Denmark, S. E.; Fan, Y. J. Org. Chem. 2005, 70, 9667–76. doi:10.1021/jo050549m
- ^ Taran, Jafar; Ramazani, Ali; Joo, Sang Woo; Ślepokura, Katarzyna; Lis, Tadeusz (2014). "Synthesis of Novel a-(Acyloxy)-a-(quinolin-4-yl)acetamides by a ThreeComponent Reaction between an Isocyanide, Quinoline-4-carbaldehyde, and Arenecarboxylic Acids". Helvetica Chimica Acta. 97: 1088–1096. doi:10.1002/hlca.201300378.
- ^ Antenucci, Achille; Marra, Francesco; Dughera, Stefano (2021). "Silica gel-immobilised chiral 1, 2-benzenedisulfonimide: a Brønsted acid heterogeneous catalyst for enantioselective multicomponent Passerini reaction". RSC Advances. 11 (42): 26083–26092. Bibcode:2021RSCAd..1126083A. doi:10.1039/D1RA05297G. PMC 9037113. PMID 35479468.
- ^ The Passirini Reaction L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 ISBN 0-471-68260-8
- ^ A Flexible Six-Component Reaction To Access Constrained Depsipeptides Based on a Dihydropyridinone Core Monica Paravidino, Rachel Scheffelaar, Rob F. Schmitz, Frans J. J. de Kanter, Marinus B. Groen, Eelco Ruijter, and Romano V. A. Orru J. Org. Chem. 2007, 72, 10239–42 doi:10.1021/jo701978v
- ^ Kreye, O.; Tóth, T.; Meier, M. J. Am. Chem. Soc., 2011, 133 (6), pp 1790–1792 [1] doi:10.1021/ja1113003
