Non-aqueous phase liquid: Difference between revisions
Reworded opening paragraph and added image |
Added history section and image |
||
| Line 1: | Line 1: | ||
'''Non-aqueous phase liquids''', or '''NAPLs''', are [[Organic compound|organic]] [[liquid]] [[Contaminant|contaminants]] characterized by their relative [[Miscibility|immiscibility]] with water. The most common examples of NAPLs include [[Petroleum product|petroleum products]], [[Coal tar|coal tars]], [[Halogenation|chlorinated]] [[Solvent|solvents]], and [[Pesticide|pesticides]].<ref name=":0">{{Cite web |last=Huling |first=Scott G. |last2=Weaver |first2=James W. |title=Dense Nonaqueous Phase Liquids |url=https://nepis.epa.gov/Exe/ZyNET.exe/2000L09G.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1991+Thru+1994&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D:%5Czyfiles%5CIndex%20Data%5C91thru94%5CTxt%5C00000015%5C2000L09G.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL# |access-date=2023-10-28 |website=nepis.epa.gov |language=en}}</ref> NAPLs can be released into the environment from a variety of [[Point source pollution|point sources]] such as improper chemical disposal, leaking underground storage tanks, septic tank effluent, and percolation from spills or landfills. The movement of NAPLs throughout the subsurface environment is complex and difficult to characterize, but important to understand when determining appropriate [[Mitigation|mitigation strategies.]] |
'''Non-aqueous phase liquids''', or '''NAPLs''', are [[Organic compound|organic]] [[liquid]] [[Contaminant|contaminants]] characterized by their relative [[Miscibility|immiscibility]] with water. The most common examples of NAPLs include [[Petroleum product|petroleum products]], [[Coal tar|coal tars]], [[Halogenation|chlorinated]] [[Solvent|solvents]], and [[Pesticide|pesticides]].<ref name=":0">{{Cite web |last=Huling |first=Scott G. |last2=Weaver |first2=James W. |title=Dense Nonaqueous Phase Liquids |url=https://nepis.epa.gov/Exe/ZyNET.exe/2000L09G.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1991+Thru+1994&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D:%5Czyfiles%5CIndex%20Data%5C91thru94%5CTxt%5C00000015%5C2000L09G.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL# |access-date=2023-10-28 |website=nepis.epa.gov |language=en}}</ref> NAPLs can be released into the environment from a variety of [[Point source pollution|point sources]] such as improper chemical disposal, leaking underground storage tanks, septic tank effluent, and percolation from spills or landfills. The movement of NAPLs throughout the subsurface environment is complex and difficult to characterize, but important to understand when determining appropriate [[Mitigation|mitigation strategies.]] |
||
[[File:Underground fuel storage tank above ground - geograph.org.uk - 2193501.jpg|thumb|Underground fuel storage tank above ground. Leakage of underground storage tanks (LUSTs) are a common point-source of NAPL pollution.]] |
|||
| ⚫ | |||
== History == |
|||
=== Attitudes about groundwater contamination before 1978 === |
|||
Groundwater has been a historically important source of water for public water systems, privately owned wells, and agricultural systems for generations. For a long time, it was commonly believed that as water traveled through soil, it was stripped of impurities before it could enter groundwater storages; as a result, there wasn't much concern about contamination of the subsurface environment<ref name=":1">{{Citation |last=Hemond |first=Harold F. |title=Chapter 3 - The Subsurface Environment |date=2023-01-01 |url=https://www.sciencedirect.com/science/article/pii/B9780128222522000037 |work=Chemical Fate and Transport in the Environment (Fourth Edition) |pages=223–316 |editor-last=Hemond |editor-first=Harold F. |access-date=2023-10-28 |place=Boston |publisher=Academic Press |isbn=978-0-12-822252-2 |last2=Fechner |first2=Elizabeth J. |editor2-last=Fechner |editor2-first=Elizabeth J.}}</ref>. |
|||
In 1960, organic contaminants including petroleum hydrocarbons, coal tar derivatives, synthetic detergents, and pesticides had been noted in an extensive literature survey of groundwater contamination, which provided the first indication of NAPLs in the subsurface<ref>{{Cite book |last=Stanley |first=William E. |url=https://books.google.com/books/about/Status_of_Knowledge_of_Ground_Water_Cont.html?id=3hhSAQAAMAAJ |title=Status of Knowledge of Ground Water Contaminants |last2=Eliassen |first2=Rolf |date=1960 |publisher=Department of Civil and Sanitary Engineering, Massachusetts Institute of Technology |language=en}}</ref>. By the early 1970s, the development of [[gas chromatography]] provided a new method to detect groundwater contaminants imperceptible to the human senses, leading to the discovery and subsequent analysis of chlorinated solvents, which are currently understood as one of the most deleterious forms of NAPL<ref name=":2" />. It became understood that NAPLs are challenging both to detect and remove from the subsurface<ref name=":0" />. Because NAPLs participate in a biological chain of degradation, they produce intermediate chemicals that provide particularly acute concerns for human health<ref name=":2">{{Citation |last=McCarty |first=Perry L. |title=Groundwater Contamination by Chlorinated Solvents: History, Remediation Technologies and Strategies |date=2010 |url=https://doi.org/10.1007/978-1-4419-1401-9_1 |work=In Situ Remediation of Chlorinated Solvent Plumes |pages=1–28 |editor-last=Stroo |editor-first=H.F. |access-date=2023-11-13 |series=SERDP/ESTCP Environmental Remediation Technology |place=New York, NY |publisher=Springer |language=en |doi=10.1007/978-1-4419-1401-9_1 |isbn=978-1-4419-1401-9 |editor2-last=Ward |editor2-first=C.H.}}</ref>. |
|||
=== Expansion of groundwater contamination research after 1978 === |
|||
These health concerns became more prevalent in the public eye after the report in 1978 of soil contamination in Niagara falls related to toxic waste having been dumped into [[Love Canal]] decades prior, which led to the passage of the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) and the creation of [[Superfund|Superfund.]] This increased attention to groundwater contamination expanded research funds, and the studies that followed revealed widespread groundwater contamination in the United States. Subsequently, the understanding of transport mechanisms and the development of remediation strategies for organic contaminants, including NAPLs, have been expanded<ref name=":2" />. |
|||
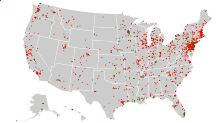
[[File:Superfund sites.svg|thumb|Map of Superfund sites in the United States. Red indicates currently on final National Priority List, yellow is proposed, green is deleted (usually meaning having been cleaned up). Data from United States Environmental Protection Agency CERCLIS database, retrieved February 12, 2015 with last update reported as October 25, 2013.]] |
|||
Early remediation strategies focused on the extraction and treatment of groundwater using well construction to restore aquifer quality (deemed the pump-and-treat strategy), but it soon became clear that the volume of water to be extracted and treated was unreasonably large, and therefore unfeasible<ref name=":2" />. Additionally, the construction of wells is invasive to the subsurface environment and can cause further infiltration of NAPLs into the bedrock, which is counter-productive<ref name=":1" />. While some experts have proposed that the complete removal of NAPLs from the subsurface environment is impossible, others view the challenge as an opportunity to expand and innovate remediation technologies<ref name=":2" />. As a result, a variety of innovations to both detect and mitigate NAPLs have been developed from the 1980s to the mid-2000s to provide alternatives to the pump-and-treat strategy<ref name=":3">{{Cite journal |last=Comegna |first=Alessandro |last2=Severino |first2=Gerardo |last3=Coppola |first3=Antonio |date=2022-10-01 |title=A review of new TDR applications for measuring non-aqueous phase liquids (NAPLs) in soils |url=https://www.sciencedirect.com/science/article/pii/S2666765722001314 |journal=Environmental Advances |volume=9 |pages=100296 |doi=10.1016/j.envadv.2022.100296 |issn=2666-7657}}</ref>.[[File:Vadose_zone.gif|thumb|An illustrated depiction of the subsurface environment. The vadose zone and the saturated zone can be distinguished by the relative abundance of liquid water, and are separated by the capillary fringe.]] |
|||
| ⚫ | NAPLs present dangers and challenges upon entering [[groundwater]], which is an important source of drinking water in addition to water used in industry and agriculture. A relatively small volume of NAPL can create toxic groundwater conditions, and NAPLs can remain in the subsurface, continually polluting groundwater, for decades or even centuries.<ref name=":1">{{Citation |last1=Hemond |first1=Harold F. |title=Chapter 3 - The Subsurface Environment |date=2023-01-01 |url=https://www.sciencedirect.com/science/article/pii/B9780128222522000037 |work=Chemical Fate and Transport in the Environment (Fourth Edition) |pages=223–316 |editor-last=Hemond |editor-first=Harold F. |access-date=2023-10-28 |place=Boston |publisher=Academic Press |isbn=978-0-12-822252-2 |last2=Fechner |first2=Elizabeth J. |editor2-last=Fechner |editor2-first=Elizabeth J.}}</ref><ref>{{Citation |last1=Henry |first1=Susan M. |title=Chlorinated Solvent and DNAPL Remediation: An Overview of Physical, Chemical, and Biological Processes |date=2002-11-10 |url=http://dx.doi.org/10.1021/bk-2002-0837.ch001 |work=ACS Symposium Series |pages=1–20 |access-date=2023-11-09 |place=Washington, DC |publisher=American Chemical Society |isbn=978-0-8412-3793-3 |last2=Hardcastle |first2=Calvin H. |last3=Warner |first3=Scott D.|doi=10.1021/bk-2002-0837.ch001 }}</ref> As such, scientists are continually developing more advanced models of NAPL behavior and strategies to detect and remove them from the subsurface environment. |
||
NAPLs can be released into the environment from a variety of [[Point source pollution|point sources]], e.g. improper chemical disposal, leaking underground storage tanks, and percolation from spills or landfills. The composition of the subsurface environment and the physical and chemical properties of the NAPLs themselves influence their movement. The subsurface can be categorized into two primary zones: the [[Vadose zone|unsaturated (vadose) zone]] and the [[Phreatic zone|saturated (phreatic) zone]], where important storages of groundwater called [[Aquifer|aquifers]] are contained. Upon entering the saturated zone, NAPLs behave differently depending on their density relative to that of water. Thus, NAPLs are typically divided into two primary types: [[Light non-aqueous phase liquid|light non-aqueous phase liquids (LNAPLs)]] and [[Dense non-aqueous phase liquid|dense non-aqueous phase liquids (DNAPLs)]]. |
NAPLs can be released into the environment from a variety of [[Point source pollution|point sources]], e.g. improper chemical disposal, leaking underground storage tanks, and percolation from spills or landfills. The composition of the subsurface environment and the physical and chemical properties of the NAPLs themselves influence their movement. The subsurface can be categorized into two primary zones: the [[Vadose zone|unsaturated (vadose) zone]] and the [[Phreatic zone|saturated (phreatic) zone]], where important storages of groundwater called [[Aquifer|aquifers]] are contained. Upon entering the saturated zone, NAPLs behave differently depending on their density relative to that of water. Thus, NAPLs are typically divided into two primary types: [[Light non-aqueous phase liquid|light non-aqueous phase liquids (LNAPLs)]] and [[Dense non-aqueous phase liquid|dense non-aqueous phase liquids (DNAPLs)]]. |
||
Revision as of 21:35, 21 November 2023
Non-aqueous phase liquids, or NAPLs, are organic liquid contaminants characterized by their relative immiscibility with water. The most common examples of NAPLs include petroleum products, coal tars, chlorinated solvents, and pesticides.[1] NAPLs can be released into the environment from a variety of point sources such as improper chemical disposal, leaking underground storage tanks, septic tank effluent, and percolation from spills or landfills. The movement of NAPLs throughout the subsurface environment is complex and difficult to characterize, but important to understand when determining appropriate mitigation strategies.

History
Attitudes about groundwater contamination before 1978
Groundwater has been a historically important source of water for public water systems, privately owned wells, and agricultural systems for generations. For a long time, it was commonly believed that as water traveled through soil, it was stripped of impurities before it could enter groundwater storages; as a result, there wasn't much concern about contamination of the subsurface environment[2].
In 1960, organic contaminants including petroleum hydrocarbons, coal tar derivatives, synthetic detergents, and pesticides had been noted in an extensive literature survey of groundwater contamination, which provided the first indication of NAPLs in the subsurface[3]. By the early 1970s, the development of gas chromatography provided a new method to detect groundwater contaminants imperceptible to the human senses, leading to the discovery and subsequent analysis of chlorinated solvents, which are currently understood as one of the most deleterious forms of NAPL[4]. It became understood that NAPLs are challenging both to detect and remove from the subsurface[1]. Because NAPLs participate in a biological chain of degradation, they produce intermediate chemicals that provide particularly acute concerns for human health[4].
Expansion of groundwater contamination research after 1978
These health concerns became more prevalent in the public eye after the report in 1978 of soil contamination in Niagara falls related to toxic waste having been dumped into Love Canal decades prior, which led to the passage of the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) and the creation of Superfund. This increased attention to groundwater contamination expanded research funds, and the studies that followed revealed widespread groundwater contamination in the United States. Subsequently, the understanding of transport mechanisms and the development of remediation strategies for organic contaminants, including NAPLs, have been expanded[4].
Early remediation strategies focused on the extraction and treatment of groundwater using well construction to restore aquifer quality (deemed the pump-and-treat strategy), but it soon became clear that the volume of water to be extracted and treated was unreasonably large, and therefore unfeasible[4]. Additionally, the construction of wells is invasive to the subsurface environment and can cause further infiltration of NAPLs into the bedrock, which is counter-productive[2]. While some experts have proposed that the complete removal of NAPLs from the subsurface environment is impossible, others view the challenge as an opportunity to expand and innovate remediation technologies[4]. As a result, a variety of innovations to both detect and mitigate NAPLs have been developed from the 1980s to the mid-2000s to provide alternatives to the pump-and-treat strategy[5].

NAPLs present dangers and challenges upon entering groundwater, which is an important source of drinking water in addition to water used in industry and agriculture. A relatively small volume of NAPL can create toxic groundwater conditions, and NAPLs can remain in the subsurface, continually polluting groundwater, for decades or even centuries.[2][6] As such, scientists are continually developing more advanced models of NAPL behavior and strategies to detect and remove them from the subsurface environment.
NAPLs can be released into the environment from a variety of point sources, e.g. improper chemical disposal, leaking underground storage tanks, and percolation from spills or landfills. The composition of the subsurface environment and the physical and chemical properties of the NAPLs themselves influence their movement. The subsurface can be categorized into two primary zones: the unsaturated (vadose) zone and the saturated (phreatic) zone, where important storages of groundwater called aquifers are contained. Upon entering the saturated zone, NAPLs behave differently depending on their density relative to that of water. Thus, NAPLs are typically divided into two primary types: light non-aqueous phase liquids (LNAPLs) and dense non-aqueous phase liquids (DNAPLs).

Depending on their densities, LNAPLs will either float on the water table upon reaching the saturated zone or pool at the bottom. As such, the mitigation strategies differ depending on the behavior of the NAPLs; mitigation of LNAPLs tends to be less complex and require simpler engineering strategies. Conversely, DNAPLs can seep into cracks in the parent material of the subsurface, complicating both their movement and the technology required for their mitigation.[2]
The composition of NAPLs is typically described using a multi-phase model that depends on parameters such as viscosity, solubility, and volatility, as well as parameters of the subsurface material including porosity, permeability, and particle size distribution. In the unsaturated zone, NAPLs can either remain as an immiscible hydrocarbon, dissolve into water, adsorb onto solid porous material, or vaporize into a gaseous form. Each of these phases differs in terms of their mobility and their available remediation techniques. This four-phase model is highly variable and can even change within a particular site during different stages of site remediation.[1] In the saturated zone, a three-phase model is utilized which excludes the gaseous phase.
A variety of parameters must be examined in order to determine the phase composition and movement of NAPL. These parameters must be understood on a microbial level in order to make decisions regarding large-scale NAPL removal, and these tests can be performed both in-situ and ex-situ. Some of these strategies tend to be invasive to the subsurface environment. Certain techniques that utilize NAPLs' chemical properties, such as time domain reflectometry which utilizes NAPLs' relative electrical permittivity, are in development to provide alternatives to these traditional techniques.[7]
References
- ^ a b c Huling, Scott G.; Weaver, James W. "Dense Nonaqueous Phase Liquids". nepis.epa.gov. Retrieved 2023-10-28.
- ^ a b c d Hemond, Harold F.; Fechner, Elizabeth J. (2023-01-01), Hemond, Harold F.; Fechner, Elizabeth J. (eds.), "Chapter 3 - The Subsurface Environment", Chemical Fate and Transport in the Environment (Fourth Edition), Boston: Academic Press, pp. 223–316, ISBN 978-0-12-822252-2, retrieved 2023-10-28 Cite error: The named reference ":1" was defined multiple times with different content (see the help page).
- ^ Stanley, William E.; Eliassen, Rolf (1960). Status of Knowledge of Ground Water Contaminants. Department of Civil and Sanitary Engineering, Massachusetts Institute of Technology.
- ^ a b c d e McCarty, Perry L. (2010), Stroo, H.F.; Ward, C.H. (eds.), "Groundwater Contamination by Chlorinated Solvents: History, Remediation Technologies and Strategies", In Situ Remediation of Chlorinated Solvent Plumes, SERDP/ESTCP Environmental Remediation Technology, New York, NY: Springer, pp. 1–28, doi:10.1007/978-1-4419-1401-9_1, ISBN 978-1-4419-1401-9, retrieved 2023-11-13
- ^ Comegna, Alessandro; Severino, Gerardo; Coppola, Antonio (2022-10-01). "A review of new TDR applications for measuring non-aqueous phase liquids (NAPLs) in soils". Environmental Advances. 9: 100296. doi:10.1016/j.envadv.2022.100296. ISSN 2666-7657.
- ^ Henry, Susan M.; Hardcastle, Calvin H.; Warner, Scott D. (2002-11-10), "Chlorinated Solvent and DNAPL Remediation: An Overview of Physical, Chemical, and Biological Processes", ACS Symposium Series, Washington, DC: American Chemical Society, pp. 1–20, doi:10.1021/bk-2002-0837.ch001, ISBN 978-0-8412-3793-3, retrieved 2023-11-09
- ^ Comegna, Alessandro; Severino, Gerardo; Coppola, Antonio (2022-10-01). "A review of new TDR applications for measuring non-aqueous phase liquids (NAPLs) in soils". Environmental Advances. 9: 100296. doi:10.1016/j.envadv.2022.100296. ISSN 2666-7657. S2CID 252577140.
