Sodium decavanadate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 24: | Line 24: | ||
}} |
}} |
||
}} |
}} |
||
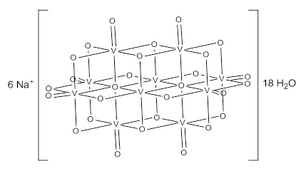
'''Sodium decavanadate''' (Na<sub>6</sub>[V<sub>10</sub>O<sub>28</sub>]) is an [[inorganic compound]]. It is the sodium salt of the decavanadate anion, [V<sub>10</sub>O<sub>28</sub>]<sup>6-</sup>. Decavanadate is unique among [[vanadates]] in that it forms an orange aqueous solution.<ref name=one>{{cite journal|authors=Johnson, G.; Murmann, R. K.; Deavin, R.; Griffith, W. P.|title=Sodium and Ammonium Decavanadates|journal= |
'''Sodium decavanadate''' (Na<sub>6</sub>[V<sub>10</sub>O<sub>28</sub>]) is an [[inorganic compound]]. It is the sodium salt of the decavanadate anion, [V<sub>10</sub>O<sub>28</sub>]<sup>6-</sup>. Decavanadate is unique among [[vanadates]] in that it forms an orange aqueous solution.<ref name=one>{{cite journal|authors=Johnson, G.; Murmann, R. K.; Deavin, R.; Griffith, W. P.|title=Sodium and Ammonium Decavanadates|journal=Inorganic Synthesese|year=2007|volume=19|pages=140-145|doi=10.1002/9780470132500.ch32}}</ref> Numerous decavanadate salts and derivatives have been isolated and studied since 1956 when it was first characterized.<ref name=two>{{cite journal|authors=Rossotti, F. J.; Rossotti, H.|title=Equilibrium Studies of Polyanions|journal=Acta Chemica Scandinavica|year=1956|volume=10|pages=957-984|doi=10.3891/acta.chem.scand.10-0957}}</ref> |
||
==Preparation== |
==Preparation== |
||
| Line 34: | Line 34: | ||
==Structure== |
==Structure== |
||
The decavanadate ion consists of six fused VO<sub>6</sub> octahedra and has D<sub>2h</sub> symmetry.<ref name=two>{{cite journal|authors=Evans Jr., H. T.|title=The molecular structure of the isopoly complex ion, decavanadate|journal=Inorg. Chem.|year=1966|volume=5|pages=967-977|doi=10.1021/ic50040a004}}</ref><ref name=three>{{cite book|authors=Kustin, K.; Pessoa, J. C.; Crans, D. C.|title=Vandadium: The Versatile Metal|year=2007|publisher=American Chemical Society|location=Washington, D. C.|isbn=978-0-8412-7446-4}}</ref> |
|||
==Acid-Base Properties== |
==Acid-Base Properties== |
||
Revision as of 17:39, 8 November 2011
| |

| |
| Identifiers | |
|---|---|
| Properties | |
| Na6[V10O28] | |
| Molar mass | 1419.6 g |
| Appearance | orange solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium decavanadate (Na6[V10O28]) is an inorganic compound. It is the sodium salt of the decavanadate anion, [V10O28]6-. Decavanadate is unique among vanadates in that it forms an orange aqueous solution.[1] Numerous decavanadate salts and derivatives have been isolated and studied since 1956 when it was first characterized.[2]
Preparation
The preparation of decavanadate is achieved by acidifying an aqueous solution of vanadate.[1]
- 10Na3[VO4]•nH2O + 24HOAc → Na6[V10O28]•18H2O + 24NaOAc
The formation of decavanadate is optimized by maintaining a pH range of 4 - 7. The product can be purified with recrystallization in water. This synthesis avoids the introduction of foreign cations, except H+. Otherwise, a variety of mixed-cation decavanadates will form. Typical side products include metavanadate, [VO3]-, and hexavanadate, [V6O16]2-, ions.[1]
Structure
The decavanadate ion consists of six fused VO6 octahedra and has D2h symmetry.[2][3]
Acid-Base Properties
Related Decavanadates
References
- ^ a b c "Sodium and Ammonium Decavanadates". Inorganic Synthesese. 19: 140–145. 2007. doi:10.1002/9780470132500.ch32.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b "Equilibrium Studies of Polyanions". Acta Chemica Scandinavica. 10: 957–984. 1956. doi:10.3891/acta.chem.scand.10-0957.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) Cite error: The named reference "two" was defined multiple times with different content (see the help page). - ^ Vandadium: The Versatile Metal. Washington, D. C.: American Chemical Society. 2007. ISBN 978-0-8412-7446-4.
{{cite book}}: Cite uses deprecated parameter|authors=(help)
