Eritoran: Difference between revisions
No edit summary |
ref |
||
| Line 28: | Line 28: | ||
| CAS_number_Ref = {{cascite|changed|??}} |
| CAS_number_Ref = {{cascite|changed|??}} |
||
| CAS_number = 185955-34-4 |
| CAS_number = 185955-34-4 |
||
| CAS_supplemental = <br />{{CAS|185954-98-7}} ( |
| CAS_supplemental = <br />{{CAS|185954-98-7}} (tetrasodium salt) |
||
| ATC_prefix = none |
| ATC_prefix = none |
||
| ATC_suffix = |
| ATC_suffix = |
||
| Line 54: | Line 54: | ||
'''Eritoran''' is an investigational drug for the treatment of severe [[sepsis]], an excessive [[inflammatory response]] to an [[infection]]. It is being developed by the Japanese pharmaceutical company [[Eisai Co.]] and administered intravenously as the [[sodium]] [[salt (chemistry)|salt]] '''eritoran tetrasodium'''.<ref>{{cite news|url=http://www.medinewsdirect.com/?p=303|title=Eritoran: A Potential Therapeutic Agent In Severe Sepsis|date=17 October 2007|publisher=MediNEWS.Direct|accessdate=26 December 2009}}</ref><ref name="PZonline">{{cite web|last=Kiemer|first=Alexandra K.|date=2008|title=TLR eröffnen neue Möglichkeiten|work=Pharmazeutische Zeitung online|publisher=Govi-Verlag|language=German|url=http://www.pharmazeutische-zeitung.de/index.php?id=4546|accessdate=26 December 2009}}</ref> |
'''Eritoran''' is an investigational drug for the treatment of severe [[sepsis]], an excessive [[inflammatory response]] to an [[infection]]. It is being developed by the Japanese pharmaceutical company [[Eisai Co.]] and administered intravenously as the [[sodium]] [[salt (chemistry)|salt]] '''eritoran tetrasodium'''.<ref>{{cite news|url=http://www.medinewsdirect.com/?p=303|title=Eritoran: A Potential Therapeutic Agent In Severe Sepsis|date=17 October 2007|publisher=MediNEWS.Direct|accessdate=26 December 2009}}</ref><ref name="PZonline">{{cite web|last=Kiemer|first=Alexandra K.|date=2008|title=TLR eröffnen neue Möglichkeiten|work=Pharmazeutische Zeitung online|publisher=Govi-Verlag|language=German|url=http://www.pharmazeutische-zeitung.de/index.php?id=4546|accessdate=26 December 2009}}</ref> |
||
In a phase III clinical trial,<ref>{{ClinicalTrialsGov|NCT00334828|ACCESS: A Controlled Comparison of Eritoran Tetrasodium and Placebo in Patients With Severe Sepsis}}</ref> eritoran did not perform better than existing treatments for the treatment of sepsis.<ref>{{cite web | title = Phase III Study for Eritoran Does Not Meet Primary Endpoint | url = http://www.drugs.com/clinical_trials/phase-iii-study-eritoran-does-not-meet-primary-endpoint-11082.html | publisher = [[drugs.com]]}}</ref> |
In a phase III clinical trial,<ref>{{ClinicalTrialsGov|NCT00334828|ACCESS: A Controlled Comparison of Eritoran Tetrasodium and Placebo in Patients With Severe Sepsis}}</ref> eritoran did not perform better than existing treatments for the treatment of sepsis.<ref>{{cite journal | doi = 10.1001/jama.2013.2194}}</ref><ref>{{cite web | title = Phase III Study for Eritoran Does Not Meet Primary Endpoint | url = http://www.drugs.com/clinical_trials/phase-iii-study-eritoran-does-not-meet-primary-endpoint-11082.html | publisher = [[drugs.com]]}}</ref> |
||
==Mechanism of action== |
==Mechanism of action== |
||
| Line 68: | Line 68: | ||
==Cytokine storm== |
==Cytokine storm== |
||
However, in influenza cases involving certain strains (involving preliminary experimentation on mice led by a [[University of Maryland School of Medicine]] researcher, not on other animals or humans), it was shown to combat another, related phenomenon called [[cytokine storm]], which can help to cause sepsis and can in concert with it or by itself can cause serious illness and death if not soon controlled, and mortality rates for sepsis, cytokine storm, and especially [[septic shock]] and organ dysfunction are still quite high despite progress made, in no small part due to the prevalence of nosocomial (hospital-acquired) infections in the ill, ongoing mutations which confer multi-drug resistance in pathological microorganisms such as bacteria and viruses (many strains of the flu are resistant to the first two drugs and some are resistant to [[Tamiflu]]), and delays in and mistakes in the recognition and treatment of disease.<ref>{{Cite web | url = http://vitals.nbcnews.com/_news/2013/05/01/18002315-new-drug-offers-novel-approach-to-taming-flu-virus | title = New drug offers novel approach to taming flu virus | publisher = NBC News}}</ref> New flu strains, such as the [[H7N9]] strain, are always emerging. |
However, in influenza cases involving certain strains (involving preliminary experimentation on mice led by a [[University of Maryland School of Medicine]] researcher, not on other animals or humans), it was shown to combat another, related phenomenon called [[cytokine storm]], which can help to cause sepsis and can in concert with it or by itself can cause serious illness and death if not soon controlled, and mortality rates for sepsis, cytokine storm, and especially [[septic shock]] and organ dysfunction are still quite high despite progress made, in no small part due to the prevalence of nosocomial (hospital-acquired) infections in the ill, ongoing mutations which confer multi-drug resistance in pathological microorganisms such as bacteria and viruses (many strains of the flu are resistant to the first two drugs and some are resistant to [[Tamiflu]]), and delays in and mistakes in the recognition and treatment of disease.<ref>{{Cite web | url = http://vitals.nbcnews.com/_news/2013/05/01/18002315-new-drug-offers-novel-approach-to-taming-flu-virus | title = New drug offers novel approach to taming flu virus | publisher = NBC News}}</ref> New flu strains, such as the [[H7N9]] strain, are always emerging. |
||
==References== |
==References== |
||
Revision as of 14:34, 3 May 2013
 | |
| Clinical data | |
|---|---|
| Other names | E 5564 |
| Routes of administration | Intravenous injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C66H126N2O19P2 |
| Molar mass | 1313.656 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Eritoran is an investigational drug for the treatment of severe sepsis, an excessive inflammatory response to an infection. It is being developed by the Japanese pharmaceutical company Eisai Co. and administered intravenously as the sodium salt eritoran tetrasodium.[1][2]
In a phase III clinical trial,[3] eritoran did not perform better than existing treatments for the treatment of sepsis.[4][5]
Mechanism of action
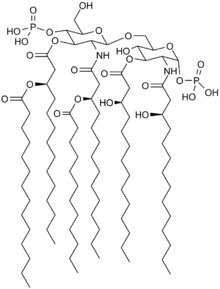
Toll-like receptors (TLRs) play an important role in the innate immune system. They recognise microbes and activate inflammatory immune responses. Toll-like receptor 4 (TLR4) detects lipopolysaccharides found in most Gram-negative bacteria.[6]
Because of its similarity to the lipopolysaccharide lipid A, eritoran acts as TLR4 antagonist and so blocks the excessive reaction triggered by this receptor.[2][7]
 |
 |
Cytokine storm
However, in influenza cases involving certain strains (involving preliminary experimentation on mice led by a University of Maryland School of Medicine researcher, not on other animals or humans), it was shown to combat another, related phenomenon called cytokine storm, which can help to cause sepsis and can in concert with it or by itself can cause serious illness and death if not soon controlled, and mortality rates for sepsis, cytokine storm, and especially septic shock and organ dysfunction are still quite high despite progress made, in no small part due to the prevalence of nosocomial (hospital-acquired) infections in the ill, ongoing mutations which confer multi-drug resistance in pathological microorganisms such as bacteria and viruses (many strains of the flu are resistant to the first two drugs and some are resistant to Tamiflu), and delays in and mistakes in the recognition and treatment of disease.[8] New flu strains, such as the H7N9 strain, are always emerging.
References
- ^ "Eritoran: A Potential Therapeutic Agent In Severe Sepsis". MediNEWS.Direct. 17 October 2007. Retrieved 26 December 2009.
- ^ a b Kiemer, Alexandra K. (2008). "TLR eröffnen neue Möglichkeiten". Pharmazeutische Zeitung online (in German). Govi-Verlag. Retrieved 26 December 2009.
- ^ Clinical trial number NCT00334828 for "ACCESS: A Controlled Comparison of Eritoran Tetrasodium and Placebo in Patients With Severe Sepsis" at ClinicalTrials.gov
- ^ . doi:10.1001/jama.2013.2194.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ "Phase III Study for Eritoran Does Not Meet Primary Endpoint". drugs.com.
- ^ "Entrez Gene: TLR4 toll-like receptor 4".
- ^ Tidswell, M; Tillis, W; Larosa, SP; Lynn, M; Wittek, AE; Kao, R; Wheeler, J; Gogate, J; Opal, SM (2010). "Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis". Critical Care Medicine. 38 (1): 72–83. doi:10.1097/CCM.0b013e3181b07b78. PMID 19661804.
- ^ "New drug offers novel approach to taming flu virus". NBC News.
