Trimesic acid: Difference between revisions
Appearance
Content deleted Content added
→See also: rm red link |
ref-format has been chaotic since the start; unifiy as CS |
||
| Line 36: | Line 36: | ||
| MeltingPt= |
| MeltingPt= |
||
| BoilingPt= |
| BoilingPt= |
||
| pKa=3.12, 3.89, 4.70<ref>Brown |
| pKa=3.12, 3.89, 4.70<ref>{{cite book |last1= Brown |first1= H.C. |author1-link= Herbert C. Brown |last2= McDaniel |first2= D.H. |last3= Häfliger |first3= O. |chapter= Chapter 14—Dissociation Constants ||editor1-last= Braude |editor1-first= E.A. |editor2-last= Nachod |editor2-first= F.C. |title= Determination of Organic Structures by Physical Methods |publisher= [[Academic Press]] |location= New York |year= 1955 |doi= 10.1016/B978-1-4832-3166-2.50018-4 }}</ref> |
||
| Solubility= |
| Solubility= |
||
}} |
}} |
||
| Line 47: | Line 47: | ||
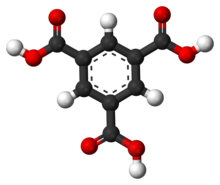
'''Trimesic acid''', also known as '''benzene-1,3,5-tricarboxylic acid''', is a [[benzene]] derivative with three [[carboxylic acid]] groups. |
'''Trimesic acid''', also known as '''benzene-1,3,5-tricarboxylic acid''', is a [[benzene]] derivative with three [[carboxylic acid]] groups. |
||
Trimesic acid is a planar molecule (and is one of only four [[benzenecarboxylic acid]]s with that property).<ref name="MARKOVIC"> |
Trimesic acid is a planar molecule (and is one of only four [[benzenecarboxylic acid]]s with that property).<ref name="MARKOVIC">{{cite journal |first1= Zoran |last1= Marković |first2= Dalibor |last2= Badjuk |first3= Ivan |last3= Gutman |year= 2004 |title= Geometry and conformations of benzenecarboxylic acids |journal= J. Serb. Chem. Soc. |volume= 69 |issue= 11 |pages= 877–882 }}</ref> |
||
ZORAN MARKOVIĆ, DALIBOR BADJUK and IVAN GUTMAN (2004), ''Geometry and conformations of benzenecarboxylic acids''. J. Serb. Chem. Soc. volume 69 issue 11, pages 877–882 (paper JSCS 3214), UDC 547.584/.585:539.193:54.02 |
|||
</ref> |
|||
Trimesic acid can be combined with [[4-Pyridone|''para''-hydroxypyridine]] to make a water-based gel, stable up to 95 °C.<ref name="Tang"> |
Trimesic acid can be combined with [[4-Pyridone|''para''-hydroxypyridine]] to make a water-based gel, stable up to 95 °C.<ref name="Tang">{{cite journal |first1= Li Ming |last1= Tang |first2= Yu Jiang |last2= Wang |year= 2009 |title= Highly stable supramolecular hydrogels formed from 1,3,5-benzenetricarboxylic acid and hydroxyl pyridines |journal= Chinese Chemical Letters |volume= 20 |issue= 10 |pages= 1259–1262 |doi= 10.1016/j.cclet.2009.04.030 }}</ref> |
||
Li Ming Tang and Yu Jiang Wang (2009), ''Highly stable supramolecular hydrogels formed from 1,3,5-benzenetricarboxylic acid and hydroxyl pyridines''. Chinese Chemical Letters volume 20, issue 10, pp. 1259–1262. {{doi|10.1016/j.cclet.2009.04.030}} |
|||
</ref> |
|||
Trimesic acid crystallizes from water in a [[hydrogen bond|hydrogen-bonded]] hydrated network with wide unidimensional empty channels.<ref name="Herbstein"> |
Trimesic acid crystallizes from water in a [[hydrogen bond|hydrogen-bonded]] hydrated network with wide unidimensional empty channels.<ref name="Herbstein">{{cite book |first= Frank H. |last= Herbstein |year= 1987 |chapter= Structural Parsimony and Structural Variety Among Inclusion Complexes (with particular reference to the inclusion compounds of trimesic acid, ''N''-(''p''-tolyl)-tetrachlorophthalimide, and the heilbron "complexes") |title= Top. Curr. Chem. |volume= 140 |pages= 107 |doi= 10.1007/bfb0003838 }}</ref> |
||
F.H. Herbstein (1987), ''Structural Parsimony and Structural Variety Among Inclusion Complexes'', Top. Curr. Chem., volume 140, p. 107 |
|||
</ref> |
|||
==See also== |
==See also== |
||
Revision as of 16:16, 16 October 2019

| |
| |
| Names | |
|---|---|
| IUPAC name
benzene-1,3,5-tricarboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.008.253 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H6O6 | |
| Molar mass | 210.14034 |
| Acidity (pKa) | 3.12, 3.89, 4.70[1] |
| Hazards | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trimesic acid, also known as benzene-1,3,5-tricarboxylic acid, is a benzene derivative with three carboxylic acid groups.
Trimesic acid is a planar molecule (and is one of only four benzenecarboxylic acids with that property).[2]
Trimesic acid can be combined with para-hydroxypyridine to make a water-based gel, stable up to 95 °C.[3]
Trimesic acid crystallizes from water in a hydrogen-bonded hydrated network with wide unidimensional empty channels.[4]
See also
References
- ^ Brown, H.C.; McDaniel, D.H.; Häfliger, O. (1955). "Chapter 14—Dissociation Constants". In Braude, E.A.; Nachod, F.C. (eds.). Determination of Organic Structures by Physical Methods. New York: Academic Press. doi:10.1016/B978-1-4832-3166-2.50018-4.
{{cite book}}: Cite has empty unknown parameter:|1=(help) - ^ Marković, Zoran; Badjuk, Dalibor; Gutman, Ivan (2004). "Geometry and conformations of benzenecarboxylic acids". J. Serb. Chem. Soc. 69 (11): 877–882.
- ^ Tang, Li Ming; Wang, Yu Jiang (2009). "Highly stable supramolecular hydrogels formed from 1,3,5-benzenetricarboxylic acid and hydroxyl pyridines". Chinese Chemical Letters. 20 (10): 1259–1262. doi:10.1016/j.cclet.2009.04.030.
- ^ Herbstein, Frank H. (1987). "Structural Parsimony and Structural Variety Among Inclusion Complexes (with particular reference to the inclusion compounds of trimesic acid, N-(p-tolyl)-tetrachlorophthalimide, and the heilbron "complexes")". Top. Curr. Chem. Vol. 140. p. 107. doi:10.1007/bfb0003838.
