Boron nitride nanotube: Difference between revisions
Citation bot (talk | contribs) m Alter: url, author. Add: url. | You can use this bot yourself. Report bugs here.| Activated by User:Nemo bis | via #UCB_webform |
MGraham300 (talk | contribs) I added info form scholar Articles about BNNTs, I also added info re Synthesis and recent developments and Also on applications Tags: use of predatory open access journal Visual edit |
||
| Line 1: | Line 1: | ||
[[File:Recovery of bent BN nanotube.jpg|thumb|upright=1.5|BN nanotube was bent inside a [[transmission electron microscope]]. Its walls self-healed after release of pressure.<ref>{{cite journal|doi=10.1039/B814607A |title=Properties and engineering of individual inorganic nanotubes in a transmission electron microscope |journal=Journal of Materials Chemistry |volume=19 |issue=7 |pages=909 |year=2009 |last1=Golberg |first1=Dmitri |last2=Costa |first2=Pedro M. F. J. |last3=Mitome |first3=Masanori |last4=Bando |first4=Yoshio }}</ref>]] |
[[File:Recovery of bent BN nanotube.jpg|thumb|upright=1.5|BN nanotube was bent inside a [[transmission electron microscope]]. Its walls self-healed after release of pressure.<ref>{{cite journal|doi=10.1039/B814607A |title=Properties and engineering of individual inorganic nanotubes in a transmission electron microscope |journal=Journal of Materials Chemistry |volume=19 |issue=7 |pages=909 |year=2009 |last1=Golberg |first1=Dmitri |last2=Costa |first2=Pedro M. F. J. |last3=Mitome |first3=Masanori |last4=Bando |first4=Yoshio }}</ref>]] |
||
'''Boron nitride nanotubes''' (BNNTs) are a [[Polymorphism (materials science)|polymorph]] of [[boron nitride]]. They were predicted in 1994<ref>{{cite journal | doi = 10.1103/PhysRevB.49.5081 | title = Theory of Graphitic Boron Nitride Nanotubes | year = 1994 | author = Rubio, A. | journal = Physical Review B | volume = 49 | page = 5081 | issue = 7 |bibcode = 1994PhRvB..49.5081R |display-authors=etal| url = https://zenodo.org/record/1233727 }}</ref> and experimentally discovered in 1995.<ref name="N.G. Chopra, R.J. Luyken 1995">{{cite journal | doi = 10.1126/science.269.5226.966 | title = Boron Nitride Nanotubes | year = 1995 | author = Chopra, N. G. | journal = Science | volume = 269 | pages = 966–7 | pmid = 17807732 | issue = 5226 |bibcode = 1995Sci...269..966C |display-authors=etal}}</ref> Structurally they are similar to [[carbon nanotubes]], which are cylinders with sub-micrometer diameters and micrometer lengths, except that carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology.<ref>{{cite journal | doi = 10.1209/0295-5075/28/5/007 | title = Stability and Band Gap Constancy of Boron Nitride Nanotubes | year = 1994 | author = Blase, X. | journal = Europhysics Letters (EPL) | volume = 28 | page = 335 | issue = 5 |bibcode = 1994EL.....28..335B |display-authors=etal| url = https://semanticscholar.org/paper/9e8cc1e698a1ed9d542b59b3bc3dbf6bd02d29db }}</ref> In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure.<ref>{{cite journal | url = http://www.glue.umd.edu/~cumings/PDF%20Publications/15.APL81han.pdf | doi = 10.1063/1.1498494 | title = Transformation of B<sub>x</sub>C<sub>y</sub>N<sub>z</sub> Nanotubes to Pure BN Nanotubes | year = 2002 | author = Wei-Qiang Han | journal = Applied Physics Letters | volume = 81 | page = 1110 | issue = 6 |bibcode = 2002ApPhL..81.1110H |display-authors=etal}}</ref><ref name=golberg>{{cite journal |author1=Golberg, D. |author2=Bando, Y. |author3=Tang, C.C. |author4=Zhi, C.Y. |lastauthoramp=yes | title = Boron Nitride Nanotubes | doi = 10.1002/adma.200700179 | journal = Advanced Materials | volume = 19 | year = 2007 | page = 2413 | issue = 18 }}</ref> |
'''Boron nitride nanotubes''' (BNNTs) are a [[Polymorphism (materials science)|polymorph]] of [[boron nitride]]. They were predicted in 1994<ref>{{cite journal | doi = 10.1103/PhysRevB.49.5081 | title = Theory of Graphitic Boron Nitride Nanotubes | year = 1994 | author = Rubio, A. | journal = Physical Review B | volume = 49 | page = 5081 | issue = 7 |bibcode = 1994PhRvB..49.5081R |display-authors=etal| url = https://zenodo.org/record/1233727 }}</ref> and experimentally discovered in 1995.<ref name="N.G. Chopra, R.J. Luyken 1995">{{cite journal | doi = 10.1126/science.269.5226.966 | title = Boron Nitride Nanotubes | year = 1995 | author = Chopra, N. G. | journal = Science | volume = 269 | pages = 966–7 | pmid = 17807732 | issue = 5226 |bibcode = 1995Sci...269..966C |display-authors=etal}}</ref> Structurally they are similar to [[carbon nanotubes]], which are cylinders with sub-micrometer diameters and micrometer lengths, except that carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology.<ref>{{cite journal | doi = 10.1209/0295-5075/28/5/007 | title = Stability and Band Gap Constancy of Boron Nitride Nanotubes | year = 1994 | author = Blase, X. | journal = Europhysics Letters (EPL) | volume = 28 | page = 335 | issue = 5 |bibcode = 1994EL.....28..335B |display-authors=etal| url = https://semanticscholar.org/paper/9e8cc1e698a1ed9d542b59b3bc3dbf6bd02d29db }}</ref> In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure.<ref>{{cite journal | url = http://www.glue.umd.edu/~cumings/PDF%20Publications/15.APL81han.pdf | doi = 10.1063/1.1498494 | title = Transformation of B<sub>x</sub>C<sub>y</sub>N<sub>z</sub> Nanotubes to Pure BN Nanotubes | year = 2002 | author = Wei-Qiang Han | journal = Applied Physics Letters | volume = 81 | page = 1110 | issue = 6 |bibcode = 2002ApPhL..81.1110H |display-authors=etal}}</ref><ref name=golberg>{{cite journal |author1=Golberg, D. |author2=Bando, Y. |author3=Tang, C.C. |author4=Zhi, C.Y. |lastauthoramp=yes | title = Boron Nitride Nanotubes | doi = 10.1002/adma.200700179 | journal = Advanced Materials | volume = 19 | year = 2007 | page = 2413 | issue = 18 }}</ref> BNNTs have unique physical and chemical properties, when compared to Carbon Nanotubes (CNTs) providing a very wide range of commercial and scientific applications <ref>{{Citation|last=Şen|first=Özlem|title=Chapter 3 - Biocompatibility evaluation of boron nitride nanotubes|date=2016-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780323389457000031|work=Boron Nitride Nanotubes in Nanomedicine|pages=41–58|editor-last=Ciofani|editor-first=Gianni|series=Micro and Nano Technologies|publisher=William Andrew Publishing|language=en|isbn=978-0-323-38945-7|access-date=2020-03-20|last2=Emanet|first2=Melis|last3=Çulha|first3=Mustafa|editor2-last=Mattoli|editor2-first=Virgilio}}</ref>. Although BNNTs and CNTs share similar tensile strength properties of circa 100 times stronger than steel and 50 times stronger than industrial-grade carbon fibre <ref>{{Cite journal|last=Jolly|first=R. D.|last2=Thompson|first2=K. G.|last3=Winchester|first3=B. G.|date=1975|title=Bovine mannosidosis--a model lysosomal storage disease|url=https://www.ncbi.nlm.nih.gov/pubmed/100|journal=Birth Defects Original Article Series|volume=11|issue=6|pages=273–278|issn=0547-6844|pmid=100}}</ref> <ref>{{Cite journal|last=Kim|first=Jun Hee|last2=Pham|first2=Thang Viet|last3=Hwang|first3=Jae Hun|last4=Kim|first4=Cheol Sang|last5=Kim|first5=Myung Jong|date=2018-06-28|title=Boron nitride nanotubes: synthesis and applications|url=https://doi.org/10.1186/s40580-018-0149-y|journal=Nano Convergence|volume=5|issue=1|pages=17|doi=10.1186/s40580-018-0149-y|issn=2196-5404|pmc=PMC6021457|pmid=30046512}}</ref>, BNNTs can withstand high temperatures of up to 900°C <ref>{{Cite web|url=https://www.sciencedaily.com/releases/2017/10/171010152901.htm|title=Step toward creating planes that travel at hypersonic speed: Air travel times could be drastically reduced by rare material|website=ScienceDaily|language=en|access-date=2020-03-20}}</ref>. as opposed to CNTs which remain stable up to temperatures of 400°C <ref>{{Cite journal|last=Mahajan|first=Amit|last2=Kingon|first2=Angus|last3=Kukovecz|first3=Ákos|last4=Konya|first4=Zoltan|last5=Vilarinho|first5=Paula M.|date=2013-01-01|title=Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres|url=http://www.sciencedirect.com/science/article/pii/S0167577X12012402|journal=Materials Letters|language=en|volume=90|pages=165–168|doi=10.1016/j.matlet.2012.08.120|issn=0167-577X}}</ref>, BNNTs are absorb Radiation and they may well help in NASA's quest to go to Mars by 2030 for their Space Radiation Shielding qualities <ref>{{Cite web|url=http://www.pitt.edu/~djm197/ENGR11_WritingAssignment3.pdf|title=RADIATION SHIELDING, BNNTS|last=|first=|date=|website=|url-status=live|archive-url=|archive-date=|access-date=}}</ref> <ref>{{Cite journal|last=Ghazizadeh|first=Mahdi|last2=Estevez|first2=Joseph|last3=Kelkar|first3=Ajit|date=2015-07-07|title=Boron Nitride Nanotubes for Space Radiation Shielding|url=https://www.researchgate.net/publication/283902943_Boron_Nitride_Nanotubes_for_Space_Radiation_Shielding|journal=International Journal of Nano Studies & Technology|pages=1–2|doi=10.19070/2167-8685-150007e}}</ref> BNNTS are packed with physicochemical features including high hydrophobicity and considerable hydrogen storage capacity and they are being investigated for possible medical and biomedical applications, including gene delivery, drug delivery, neutron capture therapy, and more generally as biomaterials <ref>{{Citation|last=Şen|first=Özlem|title=Chapter 3 - Biocompatibility evaluation of boron nitride nanotubes|date=2016-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780323389457000031|work=Boron Nitride Nanotubes in Nanomedicine|pages=41–58|editor-last=Ciofani|editor-first=Gianni|series=Micro and Nano Technologies|publisher=William Andrew Publishing|language=en|isbn=978-0-323-38945-7|access-date=2020-03-20|last2=Emanet|first2=Melis|last3=Çulha|first3=Mustafa|editor2-last=Mattoli|editor2-first=Virgilio}}</ref> BNNTs are also have superior to CNTs in they way they bond to polymers giving rise to many new applications and composite materials <ref>{{Cite web|url=https://chemical-materials.elsevier.com/new-materials-applications/cnts-are-old-stuff-make-room-for-bnnt/?fsdsd=efwe|title=CNT’s are old stuff. Make room for BNNT – it is stronger and can take the heat!|last=Elsevier|first=Name {{!}}|website=chemical-materials.elsevier.com|language=en|access-date=2020-03-20}}</ref> |
||
==Synthesis== |
==Synthesis== |
||
[[File:BN nantube RF reactor.png|thumb|upright=1.5|(a) Schematic of an [[Radio frequency|RF]] induction thermal plasma system used for mass production of BNNTs. Pure h-BN powder is continuously transformed into BNNTs by passing through a high-temperature N<sub>2</sub>-H<sub>2</sub> plasma (~8000 K). (b) Calculated temperature distribution inside the reactor. (c) Calculated velocity distribution (left) and streamlines (right).<ref name=BNpaper>{{cite journal|doi=10.1039/C5RA02988K |title=Polymer nanocomposites from free-standing, macroscopic boron nitride nanotube assemblies |journal=RSC Adv |volume=5 |issue=51 |pages=41186 |year=2015 |last1=Kim |first1=Keun Su |last2=Jakubinek |first2=Michael B. |last3=Martinez-Rubi |first3=Yadienka |last4=Ashrafi |first4=Behnam |last5=Guan |first5=Jingwen |last6=O'Neill |first6=K. |last7=Plunkett |first7=Mark |last8=Hrdina |first8=Amy |last9=Lin |first9=Shuqiong |last10=Dénommée |first10=Stéphane |last11=Kingston |first11=Christopher |last12=Simard |first12=Benoit }}</ref>]] |
[[File:BN nantube RF reactor.png|thumb|upright=1.5|(a) Schematic of an [[Radio frequency|RF]] induction thermal plasma system used for mass production of BNNTs. Pure h-BN powder is continuously transformed into BNNTs by passing through a high-temperature N<sub>2</sub>-H<sub>2</sub> plasma (~8000 K). (b) Calculated temperature distribution inside the reactor. (c) Calculated velocity distribution (left) and streamlines (right).<ref name=BNpaper>{{cite journal|doi=10.1039/C5RA02988K |title=Polymer nanocomposites from free-standing, macroscopic boron nitride nanotube assemblies |journal=RSC Adv |volume=5 |issue=51 |pages=41186 |year=2015 |last1=Kim |first1=Keun Su |last2=Jakubinek |first2=Michael B. |last3=Martinez-Rubi |first3=Yadienka |last4=Ashrafi |first4=Behnam |last5=Guan |first5=Jingwen |last6=O'Neill |first6=K. |last7=Plunkett |first7=Mark |last8=Hrdina |first8=Amy |last9=Lin |first9=Shuqiong |last10=Dénommée |first10=Stéphane |last11=Kingston |first11=Christopher |last12=Simard |first12=Benoit }}</ref>]] |
||
[[File:BN buckypaper synthesis.jpg|thumb|upright=1.5|Meters-long BN [[buckypaper]] sheets can be fabricated by adding a cylindrical drum to the above reactor.<ref name=BNpaper/>]] |
[[File:BN buckypaper synthesis.jpg|thumb|upright=1.5|Meters-long BN [[buckypaper]] sheets can be fabricated by adding a cylindrical drum to the above reactor.<ref name=BNpaper/>]] |
||
All well-established techniques of carbon nanotube growth, such as arc-discharge,<ref name="N.G. Chopra, R.J. Luyken 1995"/><ref>{{cite journal | doi = 10.1016/S0009-2614(99)01277-4 | title = Mass-Production of Boron Nitride Double-Wall Nanotubes and Nanococoons | year = 2000 | author = Cumings, J. | journal = Chemical Physics Letters | volume = 316 | page = 211 | bibcode = 2000CPL...316..211C | issue = 3–4 }}</ref> laser ablation<ref>{{cite journal | doi = 10.1063/1.116874 | title = Nanotubes in Boron Nitride Laser Heated at High Pressure | year = 1996 | author = Golberg, D. | journal = Applied Physics Letters | volume = 69 | page = 2045 | issue = 14 |bibcode = 1996ApPhL..69.2045G |display-authors=etal}}</ref><ref>{{cite journal | doi = 10.1063/1.121236 | title = Synthesis of Boron Nitride Nanotubes by Means of Excimer Laser Ablation at High Temperature | year = 1998 | author = Yu, D. P. | journal = Applied Physics Letters | volume = 72 | page = 1966 | issue = 16 |bibcode = 1998ApPhL..72.1966Y |display-authors=etal}}</ref> and chemical vapor deposition,<ref>{{cite journal | doi = 10.1016/j.ssc.2005.03.062 | title = Effective Precursor for High Yield Synthesis of Pure BN Nanotubes | year = 2005 | author = Zhi, C. | journal = Solid State Communications | volume = 135 | issue = 1–2 | page = 67 |bibcode = 2005SSCom.135...67Z |display-authors=etal}}</ref> are used for mass-production of BN nanotubes at a tens of grams scale.<ref name=BNpaper/> BN nanotubes can also be produced by [[ball mill]]ing of amorphous boron, mixed with a catalyst (iron powder), under NH<sub>3</sub> atmosphere. Subsequent annealing at ~1100 °C in nitrogen flow transforms most of the product into BN.<ref name=Au/><ref name=eu/> A high-temperature high-pressure method is also suitable for BN nanotube synthesis.<ref>{{cite journal|last1=Smith|first1=Michael W|last2=Jordan|first2=Kevin C|last3=Park|first3=Cheol|last4=Kim|first4=Jae-Woo|last5=Lillehei|first5=Peter T|last6=Crooks|first6=Roy|last7=Harrison|first7=Joycelyn S|title=Very long single- and few-walled boron nitride nanotubes via the pressurized vapor/condenser method|journal=Nanotechnology|date=16 December 2009|volume=20|issue=50|pages=505604|doi=10.1088/0957-4484/20/50/505604|pmid=19907071|bibcode = 2009Nanot..20X5604S |url=https://digital.library.unt.edu/ark:/67531/metadc845225/}}</ref> |
All well-established techniques of carbon nanotube growth, such as arc-discharge,<ref name="N.G. Chopra, R.J. Luyken 1995"/><ref>{{cite journal | doi = 10.1016/S0009-2614(99)01277-4 | title = Mass-Production of Boron Nitride Double-Wall Nanotubes and Nanococoons | year = 2000 | author = Cumings, J. | journal = Chemical Physics Letters | volume = 316 | page = 211 | bibcode = 2000CPL...316..211C | issue = 3–4 }}</ref> laser ablation<ref>{{cite journal | doi = 10.1063/1.116874 | title = Nanotubes in Boron Nitride Laser Heated at High Pressure | year = 1996 | author = Golberg, D. | journal = Applied Physics Letters | volume = 69 | page = 2045 | issue = 14 |bibcode = 1996ApPhL..69.2045G |display-authors=etal}}</ref><ref>{{cite journal | doi = 10.1063/1.121236 | title = Synthesis of Boron Nitride Nanotubes by Means of Excimer Laser Ablation at High Temperature | year = 1998 | author = Yu, D. P. | journal = Applied Physics Letters | volume = 72 | page = 1966 | issue = 16 |bibcode = 1998ApPhL..72.1966Y |display-authors=etal}}</ref> and chemical vapor deposition,<ref>{{cite journal | doi = 10.1016/j.ssc.2005.03.062 | title = Effective Precursor for High Yield Synthesis of Pure BN Nanotubes | year = 2005 | author = Zhi, C. | journal = Solid State Communications | volume = 135 | issue = 1–2 | page = 67 |bibcode = 2005SSCom.135...67Z |display-authors=etal}}</ref> are used for mass-production of BN nanotubes at a tens of grams scale.<ref name=BNpaper/> BN nanotubes can also be produced by [[ball mill]]ing of amorphous boron, mixed with a catalyst (iron powder), under NH<sub>3</sub> atmosphere. Subsequent annealing at ~1100 °C in nitrogen flow transforms most of the product into BN.<ref name=Au/><ref name=eu/> A high-temperature high-pressure method is also suitable for BN nanotube synthesis.<ref>{{cite journal|last1=Smith|first1=Michael W|last2=Jordan|first2=Kevin C|last3=Park|first3=Cheol|last4=Kim|first4=Jae-Woo|last5=Lillehei|first5=Peter T|last6=Crooks|first6=Roy|last7=Harrison|first7=Joycelyn S|title=Very long single- and few-walled boron nitride nanotubes via the pressurized vapor/condenser method|journal=Nanotechnology|date=16 December 2009|volume=20|issue=50|pages=505604|doi=10.1088/0957-4484/20/50/505604|pmid=19907071|bibcode = 2009Nanot..20X5604S |url=https://digital.library.unt.edu/ark:/67531/metadc845225/}}</ref> BNNT production route has been a significant issue due to low yield and poor quality in comparison with CNT, thus limiting its practical uses. However, many great successes in BNNT synthesis have been achieved in recent years, enabling access to this material and paving the way for the development of promising applications <ref>{{Cite journal|last=Kim|first=Jun Hee|last2=Pham|first2=Thang Viet|last3=Hwang|first3=Jae Hun|last4=Kim|first4=Cheol Sang|last5=Kim|first5=Myung Jong|date=2018-06-28|title=Boron nitride nanotubes: synthesis and applications|url=https://doi.org/10.1186/s40580-018-0149-y|journal=Nano Convergence|volume=5|issue=1|pages=17|doi=10.1186/s40580-018-0149-y|issn=2196-5404|pmc=PMC6021457|pmid=30046512}}</ref> Recently significant advancement have been made by Deakin University Australia with a ‘novel and scalable’ manufacturing process will allow the production of BNNTs in large quantities for the first time since the material was first discovered two decades ago <ref>{{Cite web|url=https://www.australianmanufacturing.com.au/43852/deakin-researchers-achieve-world-first-bnnt-breakthrough|title=Deakin researchers achieve world-first BNNT breakthrough|last=Jasmina|date=2017-03-05|website=Australian Manufacturing|language=en-US|access-date=2020-03-20}}</ref>. |
||
==Properties and potential applications== |
==Properties and potential applications== |
||
| Line 16: | Line 16: | ||
BN nanotubes have also shown potential in certain cancer treatments.<ref>{{cite journal|last1=Zhong|first1=J|last2=Dai|first2=L.C.|title=Targeting Liposomal Nanomedicine to Cancer Therapy|journal=Technology in Cancer Research & Treatment|volume=11|issue=5|pages=475–481|date=2012|doi=10.7785/tcrt.2012.500259|pmid=22475065|doi-access=free}}</ref>{{clarify |date=August 2017 |reason=the source does note cite BNNTs}} |
BN nanotubes have also shown potential in certain cancer treatments.<ref>{{cite journal|last1=Zhong|first1=J|last2=Dai|first2=L.C.|title=Targeting Liposomal Nanomedicine to Cancer Therapy|journal=Technology in Cancer Research & Treatment|volume=11|issue=5|pages=475–481|date=2012|doi=10.7785/tcrt.2012.500259|pmid=22475065|doi-access=free}}</ref>{{clarify |date=August 2017 |reason=the source does note cite BNNTs}} |
||
High stiffness and excellent chemical stability makes BNNTs ideal material for reinforcement in polymers, ceramics and metals. For instance, buckypaper-based BNNT/epoxy composites and polyurethane-modified buckypaper composites have been successfully developed.<sup>1,16</sup> These composite materials exhibit Young’s moduli over twice the value for neat epoxy and 20 times the value for unimpregnated buckypaper. BNNTs are also one of the most promising classes of material for reinforcing aluminum-based structures.<sup>17</sup> The low reactivity of BNNTs facilitates the integration of this material into an aluminum matrix where CNTs fail due to the reaction between the carbon and the aluminum which forms the undesired Al<sub>4</sub>C<sub>3</sub> phase at the interface. BNNTs also exhibit much higher oxidation temperature (~950°C) than the melting point of aluminum (660°C), which enables the homogenous dispersion of BNNTs directly into the aluminum melt. Since BNNTs retain their mechanical properties at high temperatures while having a very low density, the development of new temperature-resistant lightweight MMC is achievable. BNNTs also exhibit good thermal conductivity. This renders them useful for applications in nanoelectronics where heat dissipation is critical. This also makes BNNTs multifunctional as it not only improves the stiffness of composites but also yields high thermal conductivity along with high transparency. The combination of high stiffness and high transparency is already exploited in the development of BNNT-reinforced glass composites.<sup>18</sup> Other intrinsic properties of BNNTs such as good radiation shielding ability,<sup>19</sup> high electrical resistance and excellent piezoelectric properties are likely to promote interest for integrating them in new applications.<ref>{{Cite web|url=https://www.sigmaaldrich.com/technical-documents/articles/technology-spotlights/boron-nitride-nanotubes.html|title=Boron Nitride Nanotubes: Properties, Synthesis and Applications|website=Sigma-Aldrich|language=en|access-date=2020-03-20}}</ref> |
|||
==References== |
==References== |
||
Revision as of 05:01, 20 March 2020

Boron nitride nanotubes (BNNTs) are a polymorph of boron nitride. They were predicted in 1994[2] and experimentally discovered in 1995.[3] Structurally they are similar to carbon nanotubes, which are cylinders with sub-micrometer diameters and micrometer lengths, except that carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology.[4] In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure.[5][6] BNNTs have unique physical and chemical properties, when compared to Carbon Nanotubes (CNTs) providing a very wide range of commercial and scientific applications [7]. Although BNNTs and CNTs share similar tensile strength properties of circa 100 times stronger than steel and 50 times stronger than industrial-grade carbon fibre [8] [9], BNNTs can withstand high temperatures of up to 900°C [10]. as opposed to CNTs which remain stable up to temperatures of 400°C [11], BNNTs are absorb Radiation and they may well help in NASA's quest to go to Mars by 2030 for their Space Radiation Shielding qualities [12] [13] BNNTS are packed with physicochemical features including high hydrophobicity and considerable hydrogen storage capacity and they are being investigated for possible medical and biomedical applications, including gene delivery, drug delivery, neutron capture therapy, and more generally as biomaterials [14] BNNTs are also have superior to CNTs in they way they bond to polymers giving rise to many new applications and composite materials [15]
Synthesis


All well-established techniques of carbon nanotube growth, such as arc-discharge,[3][17] laser ablation[18][19] and chemical vapor deposition,[20] are used for mass-production of BN nanotubes at a tens of grams scale.[16] BN nanotubes can also be produced by ball milling of amorphous boron, mixed with a catalyst (iron powder), under NH3 atmosphere. Subsequent annealing at ~1100 °C in nitrogen flow transforms most of the product into BN.[21][22] A high-temperature high-pressure method is also suitable for BN nanotube synthesis.[23] BNNT production route has been a significant issue due to low yield and poor quality in comparison with CNT, thus limiting its practical uses. However, many great successes in BNNT synthesis have been achieved in recent years, enabling access to this material and paving the way for the development of promising applications [24] Recently significant advancement have been made by Deakin University Australia with a ‘novel and scalable’ manufacturing process will allow the production of BNNTs in large quantities for the first time since the material was first discovered two decades ago [25].
Properties and potential applications
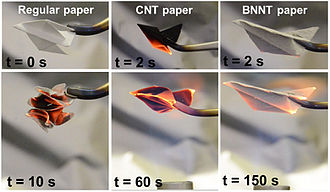
Electrical and field emission properties of BN nanotubes can be tuned by doping with gold atoms via sputtering of gold on the nanotubes.[21][26] Doping rare-earth atoms of europium turns a BN nanotube into a phosphor material emitting visible light under electron excitation.[22] Quantum dots formed from 3 nm gold particles spaced across the nanotubes exhibit the properties of field-effect transistors at room temperature.[27]
Like BN fibers, boron nitride nanotubes show promise for aerospace applications where integration of boron and in particular the light isotope of boron (10B) into structural materials improves both their strength and their radiation-shielding properties; the improvement is due to strong neutron absorption by 10B. Such 10BN materials are of particular theoretical value as composite structural materials in future manned interplanetary spacecraft, where absorption-shielding from cosmic ray spallation neutrons is expected to be a particular asset in light construction materials.[28]
Toxicological investigations on BNNTs conducted in the 2010s seem to show that the enhanced chemical inertia of BN nanotubes favors biocompatibility. As a result, their use in the biomedical field was suggested both as nanocarriers and as nanotransducers.[29]
BN nanotubes have also shown potential in certain cancer treatments.[30][clarification needed]
High stiffness and excellent chemical stability makes BNNTs ideal material for reinforcement in polymers, ceramics and metals. For instance, buckypaper-based BNNT/epoxy composites and polyurethane-modified buckypaper composites have been successfully developed.1,16 These composite materials exhibit Young’s moduli over twice the value for neat epoxy and 20 times the value for unimpregnated buckypaper. BNNTs are also one of the most promising classes of material for reinforcing aluminum-based structures.17 The low reactivity of BNNTs facilitates the integration of this material into an aluminum matrix where CNTs fail due to the reaction between the carbon and the aluminum which forms the undesired Al4C3 phase at the interface. BNNTs also exhibit much higher oxidation temperature (~950°C) than the melting point of aluminum (660°C), which enables the homogenous dispersion of BNNTs directly into the aluminum melt. Since BNNTs retain their mechanical properties at high temperatures while having a very low density, the development of new temperature-resistant lightweight MMC is achievable. BNNTs also exhibit good thermal conductivity. This renders them useful for applications in nanoelectronics where heat dissipation is critical. This also makes BNNTs multifunctional as it not only improves the stiffness of composites but also yields high thermal conductivity along with high transparency. The combination of high stiffness and high transparency is already exploited in the development of BNNT-reinforced glass composites.18 Other intrinsic properties of BNNTs such as good radiation shielding ability,19 high electrical resistance and excellent piezoelectric properties are likely to promote interest for integrating them in new applications.[31]
References
- ^ Golberg, Dmitri; Costa, Pedro M. F. J.; Mitome, Masanori; Bando, Yoshio (2009). "Properties and engineering of individual inorganic nanotubes in a transmission electron microscope". Journal of Materials Chemistry. 19 (7): 909. doi:10.1039/B814607A.
- ^ Rubio, A.; et al. (1994). "Theory of Graphitic Boron Nitride Nanotubes". Physical Review B. 49 (7): 5081. Bibcode:1994PhRvB..49.5081R. doi:10.1103/PhysRevB.49.5081.
- ^ a b Chopra, N. G.; et al. (1995). "Boron Nitride Nanotubes". Science. 269 (5226): 966–7. Bibcode:1995Sci...269..966C. doi:10.1126/science.269.5226.966. PMID 17807732.
- ^ Blase, X.; et al. (1994). "Stability and Band Gap Constancy of Boron Nitride Nanotubes". Europhysics Letters (EPL). 28 (5): 335. Bibcode:1994EL.....28..335B. doi:10.1209/0295-5075/28/5/007.
- ^ Wei-Qiang Han; et al. (2002). "Transformation of BxCyNz Nanotubes to Pure BN Nanotubes" (PDF). Applied Physics Letters. 81 (6): 1110. Bibcode:2002ApPhL..81.1110H. doi:10.1063/1.1498494.
- ^ Golberg, D.; Bando, Y.; Tang, C.C.; Zhi, C.Y. (2007). "Boron Nitride Nanotubes". Advanced Materials. 19 (18): 2413. doi:10.1002/adma.200700179.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Şen, Özlem; Emanet, Melis; Çulha, Mustafa (2016-01-01), Ciofani, Gianni; Mattoli, Virgilio (eds.), "Chapter 3 - Biocompatibility evaluation of boron nitride nanotubes", Boron Nitride Nanotubes in Nanomedicine, Micro and Nano Technologies, William Andrew Publishing, pp. 41–58, ISBN 978-0-323-38945-7, retrieved 2020-03-20
- ^ Jolly, R. D.; Thompson, K. G.; Winchester, B. G. (1975). "Bovine mannosidosis--a model lysosomal storage disease". Birth Defects Original Article Series. 11 (6): 273–278. ISSN 0547-6844. PMID 100.
- ^ Kim, Jun Hee; Pham, Thang Viet; Hwang, Jae Hun; Kim, Cheol Sang; Kim, Myung Jong (2018-06-28). "Boron nitride nanotubes: synthesis and applications". Nano Convergence. 5 (1): 17. doi:10.1186/s40580-018-0149-y. ISSN 2196-5404. PMC 6021457. PMID 30046512.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ "Step toward creating planes that travel at hypersonic speed: Air travel times could be drastically reduced by rare material". ScienceDaily. Retrieved 2020-03-20.
- ^ Mahajan, Amit; Kingon, Angus; Kukovecz, Ákos; Konya, Zoltan; Vilarinho, Paula M. (2013-01-01). "Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres". Materials Letters. 90: 165–168. doi:10.1016/j.matlet.2012.08.120. ISSN 0167-577X.
- ^ "RADIATION SHIELDING, BNNTS" (PDF).
{{cite web}}: CS1 maint: url-status (link) - ^ Ghazizadeh, Mahdi; Estevez, Joseph; Kelkar, Ajit (2015-07-07). "Boron Nitride Nanotubes for Space Radiation Shielding". International Journal of Nano Studies & Technology: 1–2. doi:10.19070/2167-8685-150007e.
- ^ Şen, Özlem; Emanet, Melis; Çulha, Mustafa (2016-01-01), Ciofani, Gianni; Mattoli, Virgilio (eds.), "Chapter 3 - Biocompatibility evaluation of boron nitride nanotubes", Boron Nitride Nanotubes in Nanomedicine, Micro and Nano Technologies, William Andrew Publishing, pp. 41–58, ISBN 978-0-323-38945-7, retrieved 2020-03-20
- ^ Elsevier, Name |. "CNT's are old stuff. Make room for BNNT – it is stronger and can take the heat!". chemical-materials.elsevier.com. Retrieved 2020-03-20.
- ^ a b c d Kim, Keun Su; Jakubinek, Michael B.; Martinez-Rubi, Yadienka; Ashrafi, Behnam; Guan, Jingwen; O'Neill, K.; Plunkett, Mark; Hrdina, Amy; Lin, Shuqiong; Dénommée, Stéphane; Kingston, Christopher; Simard, Benoit (2015). "Polymer nanocomposites from free-standing, macroscopic boron nitride nanotube assemblies". RSC Adv. 5 (51): 41186. doi:10.1039/C5RA02988K.
- ^ Cumings, J. (2000). "Mass-Production of Boron Nitride Double-Wall Nanotubes and Nanococoons". Chemical Physics Letters. 316 (3–4): 211. Bibcode:2000CPL...316..211C. doi:10.1016/S0009-2614(99)01277-4.
- ^ Golberg, D.; et al. (1996). "Nanotubes in Boron Nitride Laser Heated at High Pressure". Applied Physics Letters. 69 (14): 2045. Bibcode:1996ApPhL..69.2045G. doi:10.1063/1.116874.
- ^ Yu, D. P.; et al. (1998). "Synthesis of Boron Nitride Nanotubes by Means of Excimer Laser Ablation at High Temperature". Applied Physics Letters. 72 (16): 1966. Bibcode:1998ApPhL..72.1966Y. doi:10.1063/1.121236.
- ^ Zhi, C.; et al. (2005). "Effective Precursor for High Yield Synthesis of Pure BN Nanotubes". Solid State Communications. 135 (1–2): 67. Bibcode:2005SSCom.135...67Z. doi:10.1016/j.ssc.2005.03.062.
- ^ a b Chen, H.; et al. (2008). "Nano Au-Decorated Boron Nitride Nanotubes: Conductance Modification and Field-Emission Enhancement" (PDF). Applied Physics Letters. 92 (24): 243105. Bibcode:2008ApPhL..92x3105C. doi:10.1063/1.2943653. Archived from the original (PDF) on 2011-07-20.
- ^ a b Chen, H.; et al. (2007). "Eu-Doped Boron Nitride Nanotubes as a Nanometer-Sized Visible-Light Source" (PDF). Advanced Materials. 19 (14): 1845. doi:10.1002/adma.200700493. Archived from the original (PDF) on 2011-07-20.
- ^ Smith, Michael W; Jordan, Kevin C; Park, Cheol; Kim, Jae-Woo; Lillehei, Peter T; Crooks, Roy; Harrison, Joycelyn S (16 December 2009). "Very long single- and few-walled boron nitride nanotubes via the pressurized vapor/condenser method". Nanotechnology. 20 (50): 505604. Bibcode:2009Nanot..20X5604S. doi:10.1088/0957-4484/20/50/505604. PMID 19907071.
- ^ Kim, Jun Hee; Pham, Thang Viet; Hwang, Jae Hun; Kim, Cheol Sang; Kim, Myung Jong (2018-06-28). "Boron nitride nanotubes: synthesis and applications". Nano Convergence. 5 (1): 17. doi:10.1186/s40580-018-0149-y. ISSN 2196-5404. PMC 6021457. PMID 30046512.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Jasmina (2017-03-05). "Deakin researchers achieve world-first BNNT breakthrough". Australian Manufacturing. Retrieved 2020-03-20.
- ^ Chen, Y.; et al. (2008). "Au Doped BN Nanotubes with Tunable Conductivity". Nano. 2 (6): 367. doi:10.1142/S1793292007000702.
- ^ Lee, C. H.; Qin, S.; Savaikar, M. A.; Wang, J.; Hao, B.; Zhang, D.; Banyai, D.; Jaszczak, J. A.; Clark, K. W.; Idrobo, J. C.; Li, A. P.; Yap, Y. K. (2013). "Room-Temperature Tunneling Behavior of Boron Nitride Nanotubes Functionalized with Gold Quantum Dots". Advanced Materials. 25 (33): 4544–8. doi:10.1002/adma.201301339. PMID 23775671.
- ^ Yu, J.; et al. (2006). "Isotopically Enriched 10BN Nanotubes" (PDF). Advanced Materials. 18 (16): 2157. doi:10.1002/adma.200600231. Archived from the original (PDF) on 2011-07-17.
- ^ Ciofani, Gianni; Danti, Serena; Genchi, Giada Graziana; Mazzolai, Barbara; Mattoli, Virgilio (2013-05-27). "Boron Nitride Nanotubes: Biocompatibility and Potential Spill-Over in Nanomedicine". Small. 9 (9–10): 1672–85. doi:10.1002/smll.201201315. PMID 23423826.
- ^ Zhong, J; Dai, L.C. (2012). "Targeting Liposomal Nanomedicine to Cancer Therapy". Technology in Cancer Research & Treatment. 11 (5): 475–481. doi:10.7785/tcrt.2012.500259. PMID 22475065.
- ^ "Boron Nitride Nanotubes: Properties, Synthesis and Applications". Sigma-Aldrich. Retrieved 2020-03-20.
