Proximity labeling: Difference between revisions
m rm comment |
Citation bot (talk | contribs) Alter: title, doi, template type, journal. Add: bibcode, volume, chapter, pmc, pmid. Removed URL that duplicated unique identifier. Removed parameters. Some additions/deletions were actually parameter name changes. | You can use this bot yourself. Report bugs here. | Activated by Headbomb | via #UCB_webform |
||
| Line 1: | Line 1: | ||
[[File:Proximity_labeling_proteomics_of_mitochondrial_outer_membrane.png|thumb|Mitochondrial outer membrane proteins are identified via proximity labeling.]] |
[[File:Proximity_labeling_proteomics_of_mitochondrial_outer_membrane.png|thumb|Mitochondrial outer membrane proteins are identified via proximity labeling.]] |
||
Enzyme-catalyzed '''proximity labeling''' ('''PL'''), also known as '''proximity-based labeling''', is a [[laboratory technique]] that labels [[biomolecule]]s, usually [[protein]]s or [[RNA]], proximal to a protein of interest.<ref name=":0">{{Cite journal|last=Roux|first=Kyle J.|last2=Kim|first2=Dae In|last3=Raida|first3=Manfred|last4=Burke|first4=Brian|date=2012-03-19|title=A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells |
Enzyme-catalyzed '''proximity labeling''' ('''PL'''), also known as '''proximity-based labeling''', is a [[laboratory technique]] that labels [[biomolecule]]s, usually [[protein]]s or [[RNA]], proximal to a protein of interest.<ref name=":0">{{Cite journal|last=Roux|first=Kyle J.|last2=Kim|first2=Dae In|last3=Raida|first3=Manfred|last4=Burke|first4=Brian|date=2012-03-19|title=A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells|journal=Journal of Cell Biology|language=en|volume=196|issue=6|pages=801–810|doi=10.1083/jcb.201112098|pmid=22412018|pmc=3308701|issn=0021-9525}}</ref> By creating a [[gene fusion]] in a living cell between the protein of interest and an engineered labeling enzyme, biomolecules spatially proximal to the protein of interest can then be selectively marked with [[biotin]] for [[pulldown]] and analysis. Proximity labeling has been used for identifying the components of novel cellular structures and for determining [[Protein–protein interaction|protein-protein interaction]] partners, among other applications.<ref name=":1">{{Cite journal|last=Han|first=Shuo|last2=Li|first2=Jiefu|last3=Ting|first3=Alice Y|date=2018-06-01|title=Proximity labeling: spatially resolved proteomic mapping for neurobiology|url=http://www.sciencedirect.com/science/article/pii/S0959438817302350|journal=Current Opinion in Neurobiology|series=Neurotechnologies|language=en|volume=50|pages=17–23|doi=10.1016/j.conb.2017.10.015|pmid=29125959|pmc=6726430|issn=0959-4388}}</ref> |
||
== Principles == |
== Principles == |
||
| Line 6: | Line 6: | ||
Proximity labeling relies on a labeling enzyme that can [[Biotinylation|biotinylate]] nearby biomolecules promiscuously. Biotin labeling can be achieved through several different methods, depending on the species of labeling enzyme. |
Proximity labeling relies on a labeling enzyme that can [[Biotinylation|biotinylate]] nearby biomolecules promiscuously. Biotin labeling can be achieved through several different methods, depending on the species of labeling enzyme. |
||
* BioID is a mutant [[Escherichia coli|''E. coli'']] biotin ligase that catalyzes the [[activation]] of biotin by ATP. The activated biotin is short-lived and thus can only diffuse to a region proximal to BioID. Labeling is achieved when the activated biotin reacts with nearby [[amine]]s, such as the [[lysine]] sidechain amines found in proteins.<ref name=":0" /> TurboID is a biotin ligase engineered via [[Yeast display|yeast surface display]] [[directed evolution]]. TurboID enables ~10 minute labeling times instead of the ~18 hour labeling times required by BioID.<ref>{{Cite journal|last=Branon|first=Tess C.|last2=Bosch|first2=Justin A.|last3=Sanchez|first3=Ariana D.|last4=Udeshi|first4=Namrata D.|last5=Svinkina|first5=Tanya|last6=Carr|first6=Steven A.|last7=Feldman|first7=Jessica L.|last8=Perrimon|first8=Norbert|last9=Ting|first9=Alice Y.|date=2018-10-01|title=Efficient proximity labeling in living cells and organisms with TurboID |
* BioID is a mutant [[Escherichia coli|''E. coli'']] biotin ligase that catalyzes the [[activation]] of biotin by ATP. The activated biotin is short-lived and thus can only diffuse to a region proximal to BioID. Labeling is achieved when the activated biotin reacts with nearby [[amine]]s, such as the [[lysine]] sidechain amines found in proteins.<ref name=":0" /> TurboID is a biotin ligase engineered via [[Yeast display|yeast surface display]] [[directed evolution]]. TurboID enables ~10 minute labeling times instead of the ~18 hour labeling times required by BioID.<ref>{{Cite journal|last=Branon|first=Tess C.|last2=Bosch|first2=Justin A.|last3=Sanchez|first3=Ariana D.|last4=Udeshi|first4=Namrata D.|last5=Svinkina|first5=Tanya|last6=Carr|first6=Steven A.|last7=Feldman|first7=Jessica L.|last8=Perrimon|first8=Norbert|last9=Ting|first9=Alice Y.|date=2018-10-01|title=Efficient proximity labeling in living cells and organisms with TurboID|journal=Nature Biotechnology|language=en|volume=36|issue=9|pages=880–887|doi=10.1038/nbt.4201|pmid=30125270|pmc=6126969|issn=1546-1696}}</ref> |
||
* APEX is an [[ascorbate peroxidase]] derivative reliant on [[hydrogen peroxide]] for catalyzing the oxidation of biotin-tyramide, also known as biotin-phenol, to a short-lived and reactive biotin-phenol [[Radical (chemistry)|free radical]]. Labeling is achieved when this intermediate reacts with various functional groups of nearby biomolecules. APEX can also be used for local deposition of diaminobenzidine, a precursor for an [[Electron microscope#Sample preparation|electron microscopy stain]]. APEX2 is a derivative of APEX engineered via yeast surface display directed evolution. APEX2 shows improved labeling efficiency and cellular expression levels.<ref name=":2">{{ |
* APEX is an [[ascorbate peroxidase]] derivative reliant on [[hydrogen peroxide]] for catalyzing the oxidation of biotin-tyramide, also known as biotin-phenol, to a short-lived and reactive biotin-phenol [[Radical (chemistry)|free radical]]. Labeling is achieved when this intermediate reacts with various functional groups of nearby biomolecules. APEX can also be used for local deposition of diaminobenzidine, a precursor for an [[Electron microscope#Sample preparation|electron microscopy stain]]. APEX2 is a derivative of APEX engineered via yeast surface display directed evolution. APEX2 shows improved labeling efficiency and cellular expression levels.<ref name=":2">{{Cite book|last=Kalocsay|first=Marian|title=Proximity Labeling|chapter=APEX Peroxidase-Catalyzed Proximity Labeling and Multiplexed Quantitative Proteomics|date=2019|work=Proximity Labeling: Methods and Protocols|pages=41–55|editor-last=Sunbul|editor-first=Murat|series=Methods in Molecular Biology|volume=2008|publisher=Springer|language=en|doi=10.1007/978-1-4939-9537-0_4|pmid=31124087|isbn=978-1-4939-9537-0|editor2-last=Jäschke|editor2-first=Andres}}</ref> |
||
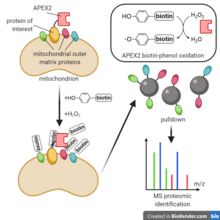
To label proteins nearby a protein of interest, a typical proximity labeling experiment begins by [[Gene expression|cellular expression]] of an APEX2 fusion to the protein of interest, which localizes to the protein of interest's native environment. Cells are next incubated with biotin-phenol, then briefly with hydrogen peroxide, initiating biotin-phenol free radical generation and labeling. To minimize cellular damage, the reaction is then quenched using an antioxidant buffer. Cells are lysed and the labeled proteins are pulled down with [[streptavidin]] beads. The proteins are digested with [[trypsin]], and finally the resulting peptidic fragments are analyzed using [[Shotgun proteomics#Workflow|shotgun proteomics]] methods such as LC-MS/MS or SPS-MS<sup>3</sup>.<ref name=":2" /> |
To label proteins nearby a protein of interest, a typical proximity labeling experiment begins by [[Gene expression|cellular expression]] of an APEX2 fusion to the protein of interest, which localizes to the protein of interest's native environment. Cells are next incubated with biotin-phenol, then briefly with hydrogen peroxide, initiating biotin-phenol free radical generation and labeling. To minimize cellular damage, the reaction is then quenched using an antioxidant buffer. Cells are lysed and the labeled proteins are pulled down with [[streptavidin]] beads. The proteins are digested with [[trypsin]], and finally the resulting peptidic fragments are analyzed using [[Shotgun proteomics#Workflow|shotgun proteomics]] methods such as LC-MS/MS or SPS-MS<sup>3</sup>.<ref name=":2" /> |
||
If instead a protein fusion is not genetically accessible (such as in human tissue samples) but an antibody for the protein of interest is known, proximity labeling can still be enabled by fusing a labeling enzyme with the antibody, then incubating the fusion with the sample.<ref>{{Cite journal|last=Rees|first=Johanna S.|last2=Li|first2=Xue-Wen|last3=Perrett|first3=Sarah|last4=Lilley|first4=Kathryn S.|last5=Jackson|first5=Antony P.|date=2015-11-01|title=Protein Neighbors and Proximity Proteomics |
If instead a protein fusion is not genetically accessible (such as in human tissue samples) but an antibody for the protein of interest is known, proximity labeling can still be enabled by fusing a labeling enzyme with the antibody, then incubating the fusion with the sample.<ref>{{Cite journal|last=Rees|first=Johanna S.|last2=Li|first2=Xue-Wen|last3=Perrett|first3=Sarah|last4=Lilley|first4=Kathryn S.|last5=Jackson|first5=Antony P.|date=2015-11-01|title=Protein Neighbors and Proximity Proteomics|journal=Molecular & Cellular Proteomics|language=en|volume=14|issue=11|pages=2848–2856|doi=10.1074/mcp.R115.052902|issn=1535-9476|pmid=26355100|pmc=4638030}}</ref><ref>{{Cite journal|last=Bar|first=Daniel Z|last2=Atkatsh|first2=Kathleen|last3=Tavarez|first3=Urraca|last4=Erdos|first4=Michael R|last5=Gruenbaum|first5=Yosef|last6=Collins|first6=Francis S|date=February 2018|title=Biotinylation by antibody recognition - A method for proximity labeling|journal=Nature Methods|volume=15|issue=2|pages=127–133|doi=10.1038/nmeth.4533|issn=1548-7091|pmc=5790613|pmid=29256494}}</ref> |
||
== Applications == |
== Applications == |
||
Proximity labeling methods have been used to study the proteomes of biological structures that are otherwise difficult to isolate purely and completely, such as [[Cilium|cilia]],<ref>{{Cite journal|last=Mick|first=David U.|last2=Rodrigues|first2=Rachel B.|last3=Leib|first3=Ryan D.|last4=Adams|first4=Christopher M.|last5=Chien|first5=Allis S.|last6=Gygi|first6=Steven P.|last7=Nachury|first7=Maxence V.|date=2015-11-23|title=Proteomics of Primary Cilia by Proximity Labeling |
Proximity labeling methods have been used to study the proteomes of biological structures that are otherwise difficult to isolate purely and completely, such as [[Cilium|cilia]],<ref>{{Cite journal|last=Mick|first=David U.|last2=Rodrigues|first2=Rachel B.|last3=Leib|first3=Ryan D.|last4=Adams|first4=Christopher M.|last5=Chien|first5=Allis S.|last6=Gygi|first6=Steven P.|last7=Nachury|first7=Maxence V.|date=2015-11-23|title=Proteomics of Primary Cilia by Proximity Labeling|journal=Developmental Cell|volume=35|issue=4|pages=497–512|doi=10.1016/j.devcel.2015.10.015|issn=1878-1551|pmc=4662609|pmid=26585297}}</ref> [[Mitochondrion|mitochondria]],<ref>{{Cite journal|last=Rhee|first=Hyun-Woo|last2=Zou|first2=Peng|last3=Udeshi|first3=Namrata D.|last4=Martell|first4=Jeffrey D.|last5=Mootha|first5=Vamsi K.|last6=Carr|first6=Steven A.|last7=Ting|first7=Alice Y.|date=2013-03-15|title=Proteomic Mapping of Mitochondria in Living Cells via Spatially Restricted Enzymatic Tagging|journal=Science|language=en|volume=339|issue=6125|pages=1328–1331|doi=10.1126/science.1230593|issn=0036-8075|pmid=23371551|pmc=3916822|bibcode=2013Sci...339.1328R}}</ref> [[Chemical synapse|postsynaptic clefts]],<ref name=":1" /> [[p-bodies]], [[stress granule]]s,<ref>{{Cite journal|last=Youn|first=Ji-Young|last2=Dunham|first2=Wade H.|last3=Hong|first3=Seo Jung|last4=Knight|first4=James D.R.|last5=Bashkurov|first5=Mikhail|last6=Chen|first6=Ginny I.|last7=Bagci|first7=Halil|last8=Rathod|first8=Bhavisha|last9=MacLeod|first9=Graham|last10=Eng|first10=Simon W.M.|last11=Angers|first11=Stéphane|date=February 2018|title=High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies|journal=Molecular Cell|volume=69|issue=3|pages=517–532.e11|doi=10.1016/j.molcel.2017.12.020|pmid=29395067|issn=1097-2765}}</ref> and [[lipid droplet]]s.<ref>{{Cite journal|last=Bersuker|first=Kirill|last2=Peterson|first2=Clark W. H.|last3=To|first3=Milton|last4=Sahl|first4=Steffen J.|last5=Savikhin|first5=Victoria|last6=Grossman|first6=Elizabeth A.|last7=Nomura|first7=Daniel K.|last8=Olzmann|first8=James A.|date=2018-01-08|title=A Proximity Labeling Strategy Provides Insights into the Composition and Dynamics of Lipid Droplet Proteomes|url=http://www.sciencedirect.com/science/article/pii/S1534580717309814|journal=Developmental Cell|language=en|volume=44|issue=1|pages=97–112.e7|doi=10.1016/j.devcel.2017.11.020|pmid=29275994|issn=1534-5807}}</ref> |
||
Fusion of APEX2 with [[G protein-coupled receptor|G-protein coupled receptors]] (GPCRs) allows for both tracking GPCR signaling at a 20 second temporal resolution<ref>{{Cite journal|last=Paek|first=Jaeho|last2=Kalocsay|first2=Marian|last3=Staus|first3=Dean P.|last4=Wingler|first4=Laura|last5=Pascolutti|first5=Roberta|last6=Paulo|first6=Joao A.|last7=Gygi|first7=Steven P.|last8=Kruse|first8=Andrew C.|date=04 06, 2017|title=Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling |
Fusion of APEX2 with [[G protein-coupled receptor|G-protein coupled receptors]] (GPCRs) allows for both tracking GPCR signaling at a 20 second temporal resolution<ref>{{Cite journal|last=Paek|first=Jaeho|last2=Kalocsay|first2=Marian|last3=Staus|first3=Dean P.|last4=Wingler|first4=Laura|last5=Pascolutti|first5=Roberta|last6=Paulo|first6=Joao A.|last7=Gygi|first7=Steven P.|last8=Kruse|first8=Andrew C.|date=04 06, 2017|title=Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling|journal=Cell|volume=169|issue=2|pages=338–349.e11|doi=10.1016/j.cell.2017.03.028|issn=1097-4172|pmc=5514552|pmid=28388415}}</ref> and also identification of unknown GPCR-linked proteins.<ref>{{Cite journal|last=Lobingier|first=Braden T.|last2=Hüttenhain|first2=Ruth|last3=Eichel|first3=Kelsie|last4=Miller|first4=Kenneth B.|last5=Ting|first5=Alice Y.|last6=von Zastrow|first6=Mark|last7=Krogan|first7=Nevan J.|date=04 06, 2017|title=An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells|journal=Cell|volume=169|issue=2|pages=350–360.e12|doi=10.1016/j.cell.2017.03.022|issn=1097-4172|pmc=5616215|pmid=28388416}}</ref> |
||
Proximity labeling has also been used for [[Transcriptomics technologies|transcriptomics]] and [[Interactome|interactomics]]. [[Alice Y. Ting|Alice Ting]] and the Ting lab at [[Stanford University]] have used APEX to identify RNA localized to specific cellular compartments.<ref>{{Cite journal|last=Shields|first=Emily J.|last2=Petracovici|first2=Ana F.|last3=Bonasio|first3=Roberto|date=2019-04-15|title=lncRedibly versatile: biochemical and biological functions of long noncoding RNAs |
Proximity labeling has also been used for [[Transcriptomics technologies|transcriptomics]] and [[Interactome|interactomics]]. [[Alice Y. Ting|Alice Ting]] and the Ting lab at [[Stanford University]] have used APEX to identify RNA localized to specific cellular compartments.<ref>{{Cite journal|last=Shields|first=Emily J.|last2=Petracovici|first2=Ana F.|last3=Bonasio|first3=Roberto|date=2019-04-15|title=lncRedibly versatile: biochemical and biological functions of long noncoding RNAs|journal=Biochemical Journal|language=en|volume=476|issue=7|pages=1083–1104|doi=10.1042/BCJ20180440|pmid=30971458|pmc=6745715|issn=0264-6021}}</ref><ref>{{Cite journal|last=Fazal|first=Furqan M.|last2=Han|first2=Shuo|last3=Parker|first3=Kevin R.|last4=Kaewsapsak|first4=Pornchai|last5=Xu|first5=Jin|last6=Boettiger|first6=Alistair N.|last7=Chang|first7=Howard Y.|last8=Ting|first8=Alice Y.|date=2019-07-11|title=Atlas of Subcellular RNA Localization Revealed by APEX-Seq|url=http://www.sciencedirect.com/science/article/pii/S0092867419305550|journal=Cell|language=en|volume=178|issue=2|pages=473–490.e26|doi=10.1016/j.cell.2019.05.027|pmid=31230715|issn=0092-8674}}</ref> Proximity labeling has also been used to find interaction partners of heterodimeric [[protein phosphatases]], of the miRISC (microRNA-induced silencing complex) protein [[RNA-induced silencing complex#RISC-associated proteins|Ago2]], and of [[ribonucleoproteins]].<ref>{{Cite journal|last=Trinkle-Mulcahy|first=Laura|date=2019-01-31|title=Recent advances in proximity-based labeling methods for interactome mapping|journal=F1000Research|language=en|volume=8|pages=135|doi=10.12688/f1000research.16903.1|pmid=30774936|pmc=6357996|issn=2046-1402}}</ref> |
||
== References == |
== References == |
||
Revision as of 20:38, 9 May 2020
Enzyme-catalyzed proximity labeling (PL), also known as proximity-based labeling, is a laboratory technique that labels biomolecules, usually proteins or RNA, proximal to a protein of interest.[1] By creating a gene fusion in a living cell between the protein of interest and an engineered labeling enzyme, biomolecules spatially proximal to the protein of interest can then be selectively marked with biotin for pulldown and analysis. Proximity labeling has been used for identifying the components of novel cellular structures and for determining protein-protein interaction partners, among other applications.[2]
Principles
Proximity labeling relies on a labeling enzyme that can biotinylate nearby biomolecules promiscuously. Biotin labeling can be achieved through several different methods, depending on the species of labeling enzyme.
- BioID is a mutant E. coli biotin ligase that catalyzes the activation of biotin by ATP. The activated biotin is short-lived and thus can only diffuse to a region proximal to BioID. Labeling is achieved when the activated biotin reacts with nearby amines, such as the lysine sidechain amines found in proteins.[1] TurboID is a biotin ligase engineered via yeast surface display directed evolution. TurboID enables ~10 minute labeling times instead of the ~18 hour labeling times required by BioID.[3]
- APEX is an ascorbate peroxidase derivative reliant on hydrogen peroxide for catalyzing the oxidation of biotin-tyramide, also known as biotin-phenol, to a short-lived and reactive biotin-phenol free radical. Labeling is achieved when this intermediate reacts with various functional groups of nearby biomolecules. APEX can also be used for local deposition of diaminobenzidine, a precursor for an electron microscopy stain. APEX2 is a derivative of APEX engineered via yeast surface display directed evolution. APEX2 shows improved labeling efficiency and cellular expression levels.[4]
To label proteins nearby a protein of interest, a typical proximity labeling experiment begins by cellular expression of an APEX2 fusion to the protein of interest, which localizes to the protein of interest's native environment. Cells are next incubated with biotin-phenol, then briefly with hydrogen peroxide, initiating biotin-phenol free radical generation and labeling. To minimize cellular damage, the reaction is then quenched using an antioxidant buffer. Cells are lysed and the labeled proteins are pulled down with streptavidin beads. The proteins are digested with trypsin, and finally the resulting peptidic fragments are analyzed using shotgun proteomics methods such as LC-MS/MS or SPS-MS3.[4]
If instead a protein fusion is not genetically accessible (such as in human tissue samples) but an antibody for the protein of interest is known, proximity labeling can still be enabled by fusing a labeling enzyme with the antibody, then incubating the fusion with the sample.[5][6]
Applications
Proximity labeling methods have been used to study the proteomes of biological structures that are otherwise difficult to isolate purely and completely, such as cilia,[7] mitochondria,[8] postsynaptic clefts,[2] p-bodies, stress granules,[9] and lipid droplets.[10]
Fusion of APEX2 with G-protein coupled receptors (GPCRs) allows for both tracking GPCR signaling at a 20 second temporal resolution[11] and also identification of unknown GPCR-linked proteins.[12]
Proximity labeling has also been used for transcriptomics and interactomics. Alice Ting and the Ting lab at Stanford University have used APEX to identify RNA localized to specific cellular compartments.[13][14] Proximity labeling has also been used to find interaction partners of heterodimeric protein phosphatases, of the miRISC (microRNA-induced silencing complex) protein Ago2, and of ribonucleoproteins.[15]
References
- ^ a b Roux, Kyle J.; Kim, Dae In; Raida, Manfred; Burke, Brian (2012-03-19). "A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells". Journal of Cell Biology. 196 (6): 801–810. doi:10.1083/jcb.201112098. ISSN 0021-9525. PMC 3308701. PMID 22412018.
- ^ a b Han, Shuo; Li, Jiefu; Ting, Alice Y (2018-06-01). "Proximity labeling: spatially resolved proteomic mapping for neurobiology". Current Opinion in Neurobiology. Neurotechnologies. 50: 17–23. doi:10.1016/j.conb.2017.10.015. ISSN 0959-4388. PMC 6726430. PMID 29125959.
- ^ Branon, Tess C.; Bosch, Justin A.; Sanchez, Ariana D.; Udeshi, Namrata D.; Svinkina, Tanya; Carr, Steven A.; Feldman, Jessica L.; Perrimon, Norbert; Ting, Alice Y. (2018-10-01). "Efficient proximity labeling in living cells and organisms with TurboID". Nature Biotechnology. 36 (9): 880–887. doi:10.1038/nbt.4201. ISSN 1546-1696. PMC 6126969. PMID 30125270.
- ^ a b Kalocsay, Marian (2019). "APEX Peroxidase-Catalyzed Proximity Labeling and Multiplexed Quantitative Proteomics". In Sunbul, Murat; Jäschke, Andres (eds.). Proximity Labeling. Methods in Molecular Biology. Vol. 2008. Springer. pp. 41–55. doi:10.1007/978-1-4939-9537-0_4. ISBN 978-1-4939-9537-0. PMID 31124087.
{{cite book}}:|work=ignored (help) - ^ Rees, Johanna S.; Li, Xue-Wen; Perrett, Sarah; Lilley, Kathryn S.; Jackson, Antony P. (2015-11-01). "Protein Neighbors and Proximity Proteomics". Molecular & Cellular Proteomics. 14 (11): 2848–2856. doi:10.1074/mcp.R115.052902. ISSN 1535-9476. PMC 4638030. PMID 26355100.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bar, Daniel Z; Atkatsh, Kathleen; Tavarez, Urraca; Erdos, Michael R; Gruenbaum, Yosef; Collins, Francis S (February 2018). "Biotinylation by antibody recognition - A method for proximity labeling". Nature Methods. 15 (2): 127–133. doi:10.1038/nmeth.4533. ISSN 1548-7091. PMC 5790613. PMID 29256494.
- ^ Mick, David U.; Rodrigues, Rachel B.; Leib, Ryan D.; Adams, Christopher M.; Chien, Allis S.; Gygi, Steven P.; Nachury, Maxence V. (2015-11-23). "Proteomics of Primary Cilia by Proximity Labeling". Developmental Cell. 35 (4): 497–512. doi:10.1016/j.devcel.2015.10.015. ISSN 1878-1551. PMC 4662609. PMID 26585297.
- ^ Rhee, Hyun-Woo; Zou, Peng; Udeshi, Namrata D.; Martell, Jeffrey D.; Mootha, Vamsi K.; Carr, Steven A.; Ting, Alice Y. (2013-03-15). "Proteomic Mapping of Mitochondria in Living Cells via Spatially Restricted Enzymatic Tagging". Science. 339 (6125): 1328–1331. Bibcode:2013Sci...339.1328R. doi:10.1126/science.1230593. ISSN 0036-8075. PMC 3916822. PMID 23371551.
- ^ Youn, Ji-Young; Dunham, Wade H.; Hong, Seo Jung; Knight, James D.R.; Bashkurov, Mikhail; Chen, Ginny I.; Bagci, Halil; Rathod, Bhavisha; MacLeod, Graham; Eng, Simon W.M.; Angers, Stéphane (February 2018). "High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies". Molecular Cell. 69 (3): 517–532.e11. doi:10.1016/j.molcel.2017.12.020. ISSN 1097-2765. PMID 29395067.
- ^ Bersuker, Kirill; Peterson, Clark W. H.; To, Milton; Sahl, Steffen J.; Savikhin, Victoria; Grossman, Elizabeth A.; Nomura, Daniel K.; Olzmann, James A. (2018-01-08). "A Proximity Labeling Strategy Provides Insights into the Composition and Dynamics of Lipid Droplet Proteomes". Developmental Cell. 44 (1): 97–112.e7. doi:10.1016/j.devcel.2017.11.020. ISSN 1534-5807. PMID 29275994.
- ^ Paek, Jaeho; Kalocsay, Marian; Staus, Dean P.; Wingler, Laura; Pascolutti, Roberta; Paulo, Joao A.; Gygi, Steven P.; Kruse, Andrew C. (04 06, 2017). "Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling". Cell. 169 (2): 338–349.e11. doi:10.1016/j.cell.2017.03.028. ISSN 1097-4172. PMC 5514552. PMID 28388415.
{{cite journal}}: Check date values in:|date=(help) - ^ Lobingier, Braden T.; Hüttenhain, Ruth; Eichel, Kelsie; Miller, Kenneth B.; Ting, Alice Y.; von Zastrow, Mark; Krogan, Nevan J. (04 06, 2017). "An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells". Cell. 169 (2): 350–360.e12. doi:10.1016/j.cell.2017.03.022. ISSN 1097-4172. PMC 5616215. PMID 28388416.
{{cite journal}}: Check date values in:|date=(help) - ^ Shields, Emily J.; Petracovici, Ana F.; Bonasio, Roberto (2019-04-15). "lncRedibly versatile: biochemical and biological functions of long noncoding RNAs". Biochemical Journal. 476 (7): 1083–1104. doi:10.1042/BCJ20180440. ISSN 0264-6021. PMC 6745715. PMID 30971458.
- ^ Fazal, Furqan M.; Han, Shuo; Parker, Kevin R.; Kaewsapsak, Pornchai; Xu, Jin; Boettiger, Alistair N.; Chang, Howard Y.; Ting, Alice Y. (2019-07-11). "Atlas of Subcellular RNA Localization Revealed by APEX-Seq". Cell. 178 (2): 473–490.e26. doi:10.1016/j.cell.2019.05.027. ISSN 0092-8674. PMID 31230715.
- ^ Trinkle-Mulcahy, Laura (2019-01-31). "Recent advances in proximity-based labeling methods for interactome mapping". F1000Research. 8: 135. doi:10.12688/f1000research.16903.1. ISSN 2046-1402. PMC 6357996. PMID 30774936.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
