Plant microbiome: Difference between revisions
Epipelagic (talk | contribs) redirect Tag: New redirect |
Epipelagic (talk | contribs) start article Tag: Removed redirect |
||
| Line 1: | Line 1: | ||
Plants live in association with diverse microorganisms, collectively called the microbiome. These microbes live either inside (endosphere) or outside (episphere) of [[plant tissue]]s. Microbes play important roles in the ecology and physiology of plants.<ref name=Dastogeer2020 /> "The core plant microbiome is thought to comprise keystone microbial taxa that are important for plant fitness and established through evolutionary mechanisms of selection and enrichment of microbial taxa containing essential functions genes for the fitness of the plant holobiont."<ref name=Compant2019>Compant, S., Samad, A., Faist, H. and Sessitsch, A. (2019) "A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application". ''Journal of advanced research'', '''19''': 29_37.{{doi|10.1016/j.jare.2019.03.004}}.</ref> |
|||
#REDIRECT [[Microbiome#Plant microbiomes]] |
|||
==Overview== |
|||
[[File:Microbiome in plant ecosystem.jpg|thumb|upright=1.7|right| {{center|'''Microbiome in plant ecosystem'''}} Schematic plant and plant-associated microbiota colonizing different niches on and inside the plant tissue. All the above-ground plant parts together, called the phyllosphere, are a continuously evolving habitat due to ultraviolet (UV) radiation and altering climatic conditions. It is primarily composed of leaves. Below-ground plant parts, mainly roots, are generally influenced by soil properties. Harmful interactions affect the plant growth through pathogenic activities of some microbiota members (left side). On the other hand, beneficial microbial interactions promote plant growth (right side).<ref>Shelake, R.M., Pramanik, D. and Kim, J.Y. (2019) "Exploration of plant-microbe interactions for sustainable agriculture in CRISPR era". ''Microorganisms'', '''7'''(8): 269. {{doi|10.3390/microorganisms7080269}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref>]] |
|||
The study of the association of plants with microorganisms precedes that of the animal and human microbiomes, notably the roles of microbes in nitrogen and phosphorus uptake. The most notable examples are plant root-[[arbuscular mycorrhizal]] (AM) and legume-[[rhizobial]] symbioses, both of which greatly influence the ability of roots to uptake various nutrients from the soil. Some of these microbes cannot survive in the absence of the plant host (the ‘obligate symbionts’ including viruses, some bacteria and fungi), which provides space, oxygen, proteins, and carbohydrates to the microorganisms. The association of AM fungi with plants has been known since 1842, and over 80 % of land plants are found associated with them.<ref>{{cite journal |doi = 10.1007/s00572-004-0307-4|title = A history of research on arbuscular mycorrhiza|year = 2004|last1 = Koide|first1 = Roger T.|last2 = Mosse|first2 = Barbara|journal = Mycorrhiza|volume = 14|issue = 3|pages = 145–163|pmid = 15088135|s2cid = 1809402}}</ref> It is thought that AM fungi helped in the domestication of plants.<ref>{{cite journal |doi = 10.1126/science.1061457|title = Molecular Evidence for the Early Colonization of Land by Fungi and Plants|year = 2001|last1 = Heckman|first1 = D. S.|journal = Science|volume = 293|issue = 5532|pages = 1129–1133|pmid = 11498589|s2cid = 10127810}}</ref> Traditionally, culturable microbes have been used for plant-microbe interaction studies with the enormous unculturable microbes remain uninvestigated and consequently, our knowledge of the roles of these unculturable microbes remains largely unknown.<ref name=Dastogeer2020>Dastogeer, K.M., Tumpa, F.H., Sultana, A., Akter, M.A. and Chakraborty, A. (2020) "Plant microbiome–an account of the factors that shape community composition and diversity". ''Current Plant Biology'': 100161. {{doi|10.1016/j.cpb.2020.100161}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref> |
|||
Unraveling the types and outcomes of plant-microbe interactions has received considerable interest among ecologists, evolutionary biologists, plant biologists, and agronomists.<ref>{{cite journal |doi = 10.1016/j.tplants.2012.04.001|title = The rhizosphere microbiome and plant health|year = 2012|last1 = Berendsen|first1 = Roeland L.|last2 = Pieterse|first2 = Corné M.J.|last3 = Bakker|first3 = Peter A.H.M.|journal = Trends in Plant Science|volume = 17|issue = 8|pages = 478–486|pmid = 22564542|hdl = 1874/255269}}</ref><ref name=Bulgarelli2013>{{cite journal |doi = 10.1146/annurev-arplant-050312-120106|title = Structure and Functions of the Bacterial Microbiota of Plants|year = 2013|last1 = Bulgarelli|first1 = Davide|last2 = Schlaeppi|first2 = Klaus|last3 = Spaepen|first3 = Stijn|last4 = Van Themaat|first4 = Emiel Ver Loren|last5 = Schulze-Lefert|first5 = Paul|journal = Annual Review of Plant Biology|volume = 64|pages = 807–838|pmid = 23373698}}</ref><ref name=Turner2013>{{cite journal |doi = 10.1186/gb-2013-14-6-209|title = The plant microbiome|year = 2013|last1 = Turner|first1 = Thomas R.|last2 = James|first2 = Euan K.|last3 = Poole|first3 = Philip S.|journal = Genome Biology|volume = 14|issue = 6|page = 209|pmid = 23805896|pmc = 3706808}}</ref> Recent developments in meta-omics and the establishment of large collections of microorganisms have dramatically increased our knowledge of the plant microbiome composition and diversity. The sequencing of marker genes of entire microbial communities, referred to as metagenomics, sheds light on the phylogenetic diversity of the microbiomes of plants. It also adds to the knowledge of the major biotic and abiotic factors responsible for shaping plant microbiome community assemblages.<ref name=Bulgarelli2013 /><ref name=Dastogeer2020 /> |
|||
{{clear}} |
|||
==Plant microbiota== |
|||
[[File:The plant microbiome.jpg|thumb|upright=2|right| Diverse microbial communities of characteristic microbiota are part of plant microbiomes, and are found on the outside surfaces and in the internal tissues of the host plant, as well as in the surrounding soil.<ref name=Dastogeer2020 />]] |
|||
{{clear}} |
|||
==Rhizosphere microbiome== |
|||
[[File:Microbial consortia naturally formed on the roots of Arabidopsis thaliana.webp|thumb|upright=1.7|right| {{center|'''Microbial consortia naturally formed<br />on the roots of ''Arabidopsis thaliana'''''}} Scanning electron microscopy pictures of root surfaces from natural ''A. thaliana'' populations showing the complex [[Microbial consortium|microbial networks]] formed on roots.<br />a) Overview of an [[Arabidopsis thaliana|''A. thaliana'']] root (primary root) with numerous root hairs. b) [[Biofilm|Biofilm-forming]] bacteria. c) [[Fungal]] or [[oomycete]] [[hyphae]] surrounding the root surface. d) Primary root densely covered by [[spore]]s and [[protist]]s. e, f) [[Protist]]s, most likely belonging to the [[Bacillariophyceae]] class. g) Bacteria and [[Filamentous bacteria|bacterial filament]]s. h, i) Different bacterial individuals showing great varieties of shapes and morphological features.<ref>Hassani, M.A., Durán, P. and Hacquard, S. (2018) "Microbial interactions within the plant holobiont". ''Microbiome'', '''6'''(1): 58. {{doi|10.1186/s40168-018-0445-0}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref>]] |
|||
{{see also|Rhizosphere}} |
|||
The rhizosphere comprises the 1–10 mm zone of soil immediately surrounding the roots that is under the influence of the plant through its deposition of root exudates, mucilage and dead plant cells.<ref>{{cite journal |doi = 10.1007/s11104-008-9885-9|title = Rhizosphere: Biophysics, biogeochemistry and ecological relevance|year = 2009|last1 = Hinsinger|first1 = Philippe|last2 = Bengough|first2 = A. Glyn|last3 = Vetterlein|first3 = Doris|last4 = Young|first4 = Iain M.|journal = Plant and Soil|volume = 321|issue = 1–2|pages = 117–152|s2cid = 8997382}}</ref> A diverse array of organisms specialize in living in the rhizosphere, including bacteria, fungi, oomycetes, nematodes, algae, protozoa, viruses, and archaea.<ref>{{cite journal |doi = 10.1007/s11104-009-0013-2|title = Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots|year = 2009|last1 = Bonkowski|first1 = Michael|last2 = Villenave|first2 = Cécile|last3 = Griffiths|first3 = Bryan|journal = Plant and Soil|volume = 321|issue = 1–2|pages = 213–233|s2cid = 35701713}}</ref> The most frequently studied beneficial rhizosphere organisms are mycorrhizae, rhizobium bacteria, plant growth promoting rhizobacteria (PGPR), and biocontrol microbes. Gans and Wolinsky projected that one gram of soil could harbor more than a million distinct bacterial genomes.<ref>{{cite journal |doi = 10.1126/science.1112665|title = Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil|year = 2005|last1 = Gans|first1 = J.|last2 = Wolinsky|first2 = M.|last3 = Dunbar|first3 = J.|journal = Science|volume = 309|issue = 5739|pages = 1387–1390|pmid = 16123304|s2cid = 130269020}}</ref> İnceoğlu and Al-Soud reported 55,121 OTUs (operational taxonomic units) from the potato rhizosphere.<ref>{{cite journal |doi = 10.1371/journal.pone.0023321|title = Comparative Analysis of Bacterial Communities in a Potato Field as Determined by Pyrosequencing|year = 2011|last1 = i̇Nceoğlu|first1 = Özgül|last2 = Al-Soud|first2 = Waleed Abu|last3 = Salles|first3 = Joana Falcão|last4 = Semenov|first4 = Alexander V.|last5 = Van Elsas|first5 = Jan Dirk|journal = PLOS ONE|volume = 6|issue = 8|pages = e23321|pmid = 21886785|pmc = 3158761}}</ref> Among the prokaryotes in the rhizosphere, the most frequent bacteria are within the Acidobacteria, Proteobacteria, Planctomycetes, Actinobacteria, Bacteroidetes, and Firmicutes.<ref name=Bulgarelli2012>{{cite journal |doi = 10.1038/nature11336|title = Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota|year = 2012|last1 = Bulgarelli|first1 = Davide|last2 = Rott|first2 = Matthias|last3 = Schlaeppi|first3 = Klaus|last4 = Ver Loren Van Themaat|first4 = Emiel|last5 = Ahmadinejad|first5 = Nahal|last6 = Assenza|first6 = Federica|last7 = Rauf|first7 = Philipp|last8 = Huettel|first8 = Bruno|last9 = Reinhardt|first9 = Richard|last10 = Schmelzer|first10 = Elmon|last11 = Peplies|first11 = Joerg|last12 = Gloeckner|first12 = Frank Oliver|last13 = Amann|first13 = Rudolf|last14 = Eickhorst|first14 = Thilo|last15 = Schulze-Lefert|first15 = Paul|journal = Nature|volume = 488|issue = 7409|pages = 91–95|pmid = 22859207|s2cid = 4393146}}</ref><ref>{{cite journal |doi = 10.1111/j.1758-2229.2009.00117.x|title = Pyrosequencing reveals a contrasted bacterial diversity between oak rhizosphere and surrounding soil|year = 2010|last1 = Uroz|first1 = Stéphane|last2 = Buée|first2 = Marc|last3 = Murat|first3 = Claude|last4 = Frey-Klett|first4 = Pascale|last5 = Martin|first5 = Francis|journal = Environmental Microbiology Reports|volume = 2|issue = 2|pages = 281–288|pmid = 23766079}}</ref> In some studies, no significant differences were reported in the microbial community composition between the bulk soil (soil not attached to the plant root) and rhizosphere soil.<ref>{{cite journal |doi = 10.1038/nature11237|title = Defining the core Arabidopsis thaliana root microbiome|year = 2012|last1 = Lundberg|first1 = Derek S.|last2 = Lebeis|first2 = Sarah L.|last3 = Paredes|first3 = Sur Herrera|last4 = Yourstone|first4 = Scott|last5 = Gehring|first5 = Jase|last6 = Malfatti|first6 = Stephanie|last7 = Tremblay|first7 = Julien|last8 = Engelbrektson|first8 = Anna|last9 = Kunin|first9 = Victor|last10 = Rio|first10 = Tijana Glavina del|last11 = Edgar|first11 = Robert C.|last12 = Eickhorst|first12 = Thilo|last13 = Ley|first13 = Ruth E.|last14 = Hugenholtz|first14 = Philip|last15 = Tringe|first15 = Susannah Green|last16 = Dangl|first16 = Jeffery L.|journal = Nature|volume = 488|issue = 7409|pages = 86–90|pmid = 22859206|pmc = 4074413}}</ref><ref name=Schlaeppi2014>{{cite journal |doi = 10.1073/pnas.1321597111|title = Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives|year = 2014|last1 = Schlaeppi|first1 = K.|last2 = Dombrowski|first2 = N.|last3 = Oter|first3 = R. G.|last4 = Ver Loren Van Themaat|first4 = E.|last5 = Schulze-Lefert|first5 = P.|journal = Proceedings of the National Academy of Sciences|volume = 111|issue = 2|pages = 585–592|pmid = 24379374|s2cid = 13806811}}</ref> Certain bacterial groups (e. g. Actinobacteria, Xanthomonadaceae) are less abundant in the rhizosphere than in nearby bulk soil .<ref name=Bulgarelli2012 /><ref name=Dastogeer2020 /> |
|||
[[Mycorrhizal fungi]] are abundant members of the rhizosphere community, and have been found in over 200,000 plant species, and are estimated to associate with over 80% of all plants.<ref>{{cite journal |doi = 10.1111/nph.13288|title = Mycorrhizal ecology and evolution: The past, the present, and the future|year = 2015|last1 = Van Der Heijden|first1 = Marcel G. A.|last2 = Martin|first2 = Francis M.|last3 = Selosse|first3 = Marc-André|last4 = Sanders|first4 = Ian R.|journal = New Phytologist|volume = 205|issue = 4|pages = 1406–1423|pmid = 25639293}}</ref> These mycorrhizae–root associations play profound roles in land ecosystems by regulating nutrient and carbon cycles. Mycorrhizae are integral to plant health because they provide up to 80 % of N and P requirements. In return, the fungi obtain carbohydrates and lipids from host plants.<ref>{{cite journal |doi = 10.1016/j.tplants.2017.05.008|title = Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter?|year = 2017|last1 = Rich|first1 = Mélanie K.|last2 = Nouri|first2 = Eva|last3 = Courty|first3 = Pierre-Emmanuel|last4 = Reinhardt|first4 = Didier|journal = Trends in Plant Science|volume = 22|issue = 8|pages = 652–660|pmid = 28622919|url = http://doc.rero.ch/record/305034/files/rei_dam.pdf}}</ref> Recent studies of arbuscular mycorrhizal fungi using sequencing technologies show greater between-species and within-species diversity than previously known.<ref>{{cite journal |doi = 10.5941/MYCO.2013.41.3.121|title = Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems|year = 2013|last1 = Lee|first1 = Eun-Hwa|last2 = Eo|first2 = Ju-Kyeong|last3 = Ka|first3 = Kang-Hyeon|last4 = Eom|first4 = Ahn-Heum|journal = Mycobiology|volume = 41|issue = 3|pages = 121–125|pmid = 24198665|pmc = 3817225}}</ref><ref name=Dastogeer2020 /> |
|||
{{clear}} |
|||
==Phyllosphere microbiome== |
|||
[[File:Healthy and unhealthy leaf.png|thumb|upright=1|right| A leaf from a healthy ''[[Arabidopsis]]'' plant (left) and a leaf from a dysbiosis mutant plant (right)<ref name=He2020>[[Sheng-Yang He|He, Sheng Yang]] (2020) [https://theconversation.com/when-plants-and-their-microbes-are-not-in-sync-the-results-can-be-disastrous-143802 When plants and their microbes are not in sync, the results can be disastrous] ''The Conversation'', 28 August 2020.</ref>]] |
|||
{{see also|Phyllosphere}} |
|||
The aerial surface of a plant (stem, leaf, flower, fruit) is called the [[phyllosphere]] and is considered comparatively nutrient poor when compared to the rhizosphere and endosphere. The environment in the phyllosphere is more dynamic than the rhizosphere and endosphere environments. Microbial colonizers are subjected to diurnal and seasonal fluctuations of heat, moisture, and radiation. In addition, these environmental elements affect plant physiology (such as photosynthesis, respiration, water uptake etc.) and indirectly influence microbiome composition.<ref name=Dastogeer2020 /> Rain and wind also cause temporal variation to the phyllosphere microbiome.<ref>{{cite book |doi = 10.1007/978-0-585-34164-4_10|chapter = Role of Immigration and Other Processes in Determining Epiphytic Bacterial Populations|title = Aerial Plant Surface Microbiology|year = 1996|last1 = Lindow|first1 = Steven E.|pages = 155–168|isbn = 978-0-306-45382-3}}</ref> Overall, there remains high species richness in phyllosphere communities. Fungal communities are highly variable in the phyllosphere of temperate regions and are more diverse than in tropical regions.<ref name=Vorholt2012>{{cite journal |doi = 10.1128/AEM.05565-11|title = Geographical Location Determines the Population Structure in Phyllosphere Microbial Communities of a Salt-Excreting Desert Tree|year = 2011|last1 = Finkel|first1 = Omri M.|last2 = Burch|first2 = Adrien Y.|last3 = Lindow|first3 = Steven E.|last4 = Post|first4 = Anton F.|last5 = Belkin|first5 = Shimshon|journal = Applied and Environmental Microbiology|volume = 77|issue = 21|pages = 7647–7655|pmid = 21926212|pmc = 3209174}}</ref> There can be up to 107 microbes per cm2 present on leaf surfaces of plants, and thus the bacterial population of the phyllosphere on a global scale is estimated to be 1026 cells.<ref>{{cite journal |doi = 10.1038/nrmicro2910|title = Microbial life in the phyllosphere|year = 2012|last1 = Vorholt|first1 = Julia A.|journal = Nature Reviews Microbiology|volume = 10|issue = 12|pages = 828–840|pmid = 23154261|s2cid = 10447146}}</ref> The population size of the fungal phyllosphere is likely to be smaller [25]. Phyllosphere microbes from different plants appear to be somewhat similar at high levels of taxa, but at the lower levels taxa there remain significant differences. This indicates that microorganisms may need finely tuned metabolic adjustment to survive in phyllosphere environment.<ref name=Vorholt2012 /> Proteobacteria seems to be the dominant colonizers, with Bacteroidetes and Actinobacteria also predominant in phyllospheres.<ref>{{cite journal |doi = 10.1371/journal.pone.0056329|title = Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis thaliana|year = 2013|last1 = Bodenhausen|first1 = Natacha|last2 = Horton|first2 = Matthew W.|last3 = Bergelson|first3 = Joy|journal = PLOS ONE|volume = 8|issue = 2|pages = e56329|pmid = 23457551|pmc = 3574144}}</ref> Although there are similarities between the rhizosphere and soil microbial communities, very low similarity has been reported between phyllosphere communities and those in open air.<ref>{{cite journal |doi = 10.1007/s00248-012-0053-7|title = Exploring Biodiversity in the Bacterial Community of the Mediterranean Phyllosphere and its Relationship with Airborne Bacteria|year = 2012|last1 = Vokou|first1 = Despoina|last2 = Vareli|first2 = Katerina|last3 = Zarali|first3 = Ekaterini|last4 = Karamanoli|first4 = Katerina|last5 = Constantinidou|first5 = Helen-Isis A.|last6 = Monokrousos|first6 = Nikolaos|last7 = Halley|first7 = John M.|last8 = Sainis|first8 = Ioannis|journal = Microbial Ecology|volume = 64|issue = 3|pages = 714–724|pmid = 22544345|s2cid = 17291303}}</ref><ref name=Dastogeer2020 /> |
|||
==Endosphere microbiome== |
|||
Some microorganisms, such as endophytes, penetrate and occupy the plant internal tissues, forming the endospheric microbiome. The AM and other endophytic fungi are the dominant colonizers of the endosphere.<ref name=Dastogeer2017>{{cite journal |doi = 10.1007/s00248-012-0053-7|title = Exploring Biodiversity in the Bacterial Community of the Mediterranean Phyllosphere and its Relationship with Airborne Bacteria|year = 2012|last1 = Vokou|first1 = Despoina|last2 = Vareli|first2 = Katerina|last3 = Zarali|first3 = Ekaterini|last4 = Karamanoli|first4 = Katerina|last5 = Constantinidou|first5 = Helen-Isis A.|last6 = Monokrousos|first6 = Nikolaos|last7 = Halley|first7 = John M.|last8 = Sainis|first8 = Ioannis|journal = Microbial Ecology|volume = 64|issue = 3|pages = 714–724|pmid = 22544345|s2cid = 17291303}}</ref> Bacteria, and to some degree Archaea, are important members of endosphere communities. Some of these endophytic microbes interact with their host and provide obvious benefits to plants.<ref name=Bulgarelli2012 /><ref>{{cite journal |doi = 10.1016/j.envexpbot.2017.08.008|title = Metabolic responses of endophytic Nicotiana benthamiana plants experiencing water stress|year = 2017|last1 = Dastogeer|first1 = Khondoker M.G.|last2 = Li|first2 = Hua|last3 = Sivasithamparam|first3 = Krishnapillai|last4 = Jones|first4 = Michael G.K.|last5 = Du|first5 = Xin|last6 = Ren|first6 = Yonglin|last7 = Wylie|first7 = Stephen J.|journal = Environmental and Experimental Botany|volume = 143|pages = 59–71|url = https://researchrepository.murdoch.edu.au/id/eprint/38407/}}</ref><ref>{{cite journal |doi = 10.1111/j.1469-8137.2009.02773.x|title = Fungal endophytes: Diversity and functional roles|year = 2009|last1 = Rodriguez|first1 = R. J.|last2 = White Jr|first2 = J. F.|last3 = Arnold|first3 = A. E.|last4 = Redman|first4 = R. S.|journal = New Phytologist|volume = 182|issue = 2|pages = 314–330|pmid = 19236579}}</ref> Unlike the rhizosphere and the rhizoplane, the endospheres harbor highly specific microbial communities. The root endophytic community can be very distinct from that of the adjacent soil community. In general, diversity of the endophytic community is lower than the diversity of the microbial community outside the plant.<ref name=Schlaeppi2014 /> The identity and diversity of the endophytic microbiome of above-and below-ground tissues may also differ within the plant.<ref name=Dastogeer2017 /><ref name=Dastogeer2020 /> |
|||
==References== |
|||
{{reflist}} |
|||
==Reference books== |
|||
<small>{{div col|colwidth=30em}} |
|||
* Saleem M (2015) [https://books.google.com/books?id=8vVNBgAAQBAJ&printsec=frontcover&dq=%22Microbiome+Community+Ecology:+Fundamentals+and+Applications%22&hl=en&sa=X&ved=2ahUKEwj1ueTplZfrAhV6wjgGHd2HCd8Q6AEwAHoECAQQAg#v=onepage&q=%22Microbiome%20Community%20Ecology%3A%20Fundamentals%20and%20Applications%22&f=false ''Microbiome Community Ecology: Fundamentals and Applications''] Springer. {{ISBN|9783319116655}}. |
|||
* Kumar V, Prasad R, Kumar M and Choudhary DK (2019) [https://books.google.com/books?id=dY2oDwAAQBAJ&printsec=frontcover&dq=%22Microbiome+in+Plant+Health+and+Disease%22&hl=en&newbks=1&newbks_redir=0&sa=X&ved=2ahUKEwjVwJn90P7rAhV6ILcAHVjSDb4Q6AEwAHoECAMQAg#v=onepage&q=%22Microbiome%20in%20Plant%20Health%20and%20Disease%22&f=false ''Microbiome in Plant Health and Disease: Challenges and Opportunities''] Springer. {{ISBN|9789811384950}}. |
|||
* {{Cite book|url=https://books.google.com/books?id=dY2oDwAAQBAJ&printsec=frontcover&dq=%22Microbiome+in+Plant+Health+and+Disease%22#q=%22Microbiome%20in%20Plant%20Health%20and%20Disease%22|title=Microbiome in Plant Health and Disease: Challenges and Opportunities|isbn=9789811384950|last1=Kumar|first1=Vivek|last2=Prasad|first2=Ram|last3=Kumar|first3=Manoj|last4=Choudhary|first4=Devendra K.|date=10 August 2019}} |
|||
* {{Cite book|url=https://books.google.com/books?id=NtlpBgAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = The plant microbiome and its importance for plant and human health|isbn = 9782889193783|last1 = Grube|first1 = Martin|last2 = Schloter|first2 = Michael|last3 = Smalla|first3 = Kornelia|last4 = Berg|first4 = Gabriele|date = 22 January 2015|pmid = 25278934}} |
|||
* {{Cite book|url=https://books.google.com/books?id=-cNKDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Plant Microbiome: Stress Response|isbn = 9789811055140|last1 = Egamberdieva|first1 = Dilfuza|last2 = Ahmad|first2 = Parvaiz|date = 6 February 2018}} |
|||
* {{Cite book|url=https://books.google.com/books?id=XniqDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Effects of Plant-Microbiome Interactions on Phyto- and Bio-Remediation Capacity|isbn = 9782889459322|last1 = Castiglione|first1 = Stefano|last2 = Cicatelli|first2 = Angela|last3 = Ferrol|first3 = Nuria|last4 = Rozpadek|first4 = Piotr|date = 22 August 2019}} |
|||
* {{Cite book|url=https://books.google.com/books?id=XniqDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Effects of Plant-Microbiome Interactions on Phyto- and Bio-Remediation Capacity|isbn = 9782889459322|last1 = Castiglione|first1 = Stefano|last2 = Cicatelli|first2 = Angela|last3 = Ferrol|first3 = Nuria|last4 = Rozpadek|first4 = Piotr|date = 22 August 2019}} |
|||
* {{Cite book|url=https://books.google.com/books?id=-L6WzQEACAAJ&dq=plant+microbiome|title=The Plant Microbiome: Methods and Protocols|isbn=9781071610398|last1=Carvalhais|first1=Lilia C.|last2=Dennis|first2=Paul G.|date=30 December 2020}} |
|||
* {{Cite book|url=https://books.google.com/books?id=dY2oDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Microbiome in Plant Health and Disease: Challenges and Opportunities|isbn = 9789811384950|last1 = Kumar|first1 = Vivek|last2 = Prasad|first2 = Ram|last3 = Kumar|first3 = Manoj|last4 = Choudhary|first4 = Devendra K.|date = 10 August 2019}} |
|||
* {{Cite book|url=https://books.google.com/books?id=uujeDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Microbiomes and Plant Health: Panoply and Their Applications|isbn = 9780128226018|last1 = Solanki|first1 = Manoj Kumar|last2 = Kashyap|first2 = Prem Lal|last3 = Ansari|first3 = Rizwan Ali|last4 = Kumari|first4 = Baby|date = 28 August 2020}} |
|||
* {{Cite book|url=https://books.google.com/books?id=_6L0DwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Advances in Plant Microbiome and Sustainable Agriculture: Diversity and Biotechnological Applications|isbn = 9789811532085|last1 = Yadav|first1 = Ajar Nath|year = 2020}} |
|||
* {{Cite book|url=https://books.google.com/books?id=h_75DwAAQBAJ&pg=PA35&dq=plant+microbiome#q=plant%20microbiome|title = Plant Microbiome Paradigm|isbn = 9783030503956|last1 = Varma|first1 = Ajit}} |
|||
* {{Cite book|url=https://books.google.com/books?id=Mvo2DwAAQBAJ&pg=PA1&dq=plant+microbiome#q=plant%20microbiome|title = Functional Importance of the Plant Microbiome: Implications for Agriculture, Forestry and Bioenergy|isbn = 9783319658971|last1 = Doty|first1 = Sharon Lafferty|date = 21 September 2017}} |
|||
* {{Cite book|url=https://books.google.com/books?id=yzn1DwAAQBAJ&pg=PA87&dq=plant+microbiome#q=plant%20microbiome|title = Advances in Plant Microbiome and Sustainable Agriculture|isbn = 9789811532047|author1 = Yadav|year = 2020}} |
|||
* {{Cite book|url=https://books.google.com/books?id=GljPDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Microbiomes of Soils, Plants and Animals: An Integrated Approach|isbn = 9781108473712|last1 = Antwis|first1 = Rachael E.|last2 = Harrison|first2 = Xavier A.|last3 = Cox|first3 = Michael J.|date = 12 March 2020}} |
|||
* {{Cite book|url=https://books.google.com/books?id=d6WQzQEACAAJ&dq=plant+microbiome|title=Plant Microbiome Paradigm|isbn=9783030503949|last1=Varma|first1=Ajit|last2=Tripathi|first2=Swati|last3=Prasad|first3=Ram|date=20 October 2020}} |
|||
* {{Cite book|url=https://books.google.com/books?id=YY24BAAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = The Hologenome Concept: Human, Animal and Plant Microbiota|isbn = 9783319042411|last1 = Rosenberg|first1 = Eugene|last2 = Zilber-Rosenberg|first2 = Ilana|date = 31 January 2014}} |
|||
* {{Cite book|url=https://books.google.com/books?id=HEHVDwAAQBAJ&printsec=frontcover&dq=plant+microbiome#q=plant%20microbiome|title = Plant Microbiomes for Sustainable Agriculture|isbn = 9783030384531|last1 = Yadav|first1 = Ajar Nath|last2 = Singh|first2 = Joginder|last3 = Rastegari|first3 = Ali Asghar|last4 = Yadav|first4 = Neelam|date = 6 March 2020}} |
|||
{{div col end}}</small> |
|||
{{microorganisms}} |
|||
[[Category:Microbiomes]] |
|||
Revision as of 06:10, 29 September 2020
Plants live in association with diverse microorganisms, collectively called the microbiome. These microbes live either inside (endosphere) or outside (episphere) of plant tissues. Microbes play important roles in the ecology and physiology of plants.[1] "The core plant microbiome is thought to comprise keystone microbial taxa that are important for plant fitness and established through evolutionary mechanisms of selection and enrichment of microbial taxa containing essential functions genes for the fitness of the plant holobiont."[2]
Overview

The study of the association of plants with microorganisms precedes that of the animal and human microbiomes, notably the roles of microbes in nitrogen and phosphorus uptake. The most notable examples are plant root-arbuscular mycorrhizal (AM) and legume-rhizobial symbioses, both of which greatly influence the ability of roots to uptake various nutrients from the soil. Some of these microbes cannot survive in the absence of the plant host (the ‘obligate symbionts’ including viruses, some bacteria and fungi), which provides space, oxygen, proteins, and carbohydrates to the microorganisms. The association of AM fungi with plants has been known since 1842, and over 80 % of land plants are found associated with them.[4] It is thought that AM fungi helped in the domestication of plants.[5] Traditionally, culturable microbes have been used for plant-microbe interaction studies with the enormous unculturable microbes remain uninvestigated and consequently, our knowledge of the roles of these unculturable microbes remains largely unknown.[1]
Unraveling the types and outcomes of plant-microbe interactions has received considerable interest among ecologists, evolutionary biologists, plant biologists, and agronomists.[6][7][8] Recent developments in meta-omics and the establishment of large collections of microorganisms have dramatically increased our knowledge of the plant microbiome composition and diversity. The sequencing of marker genes of entire microbial communities, referred to as metagenomics, sheds light on the phylogenetic diversity of the microbiomes of plants. It also adds to the knowledge of the major biotic and abiotic factors responsible for shaping plant microbiome community assemblages.[7][1]
Plant microbiota
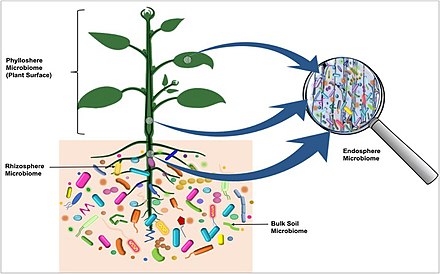
Rhizosphere microbiome

on the roots of Arabidopsis thaliana
a) Overview of an A. thaliana root (primary root) with numerous root hairs. b) Biofilm-forming bacteria. c) Fungal or oomycete hyphae surrounding the root surface. d) Primary root densely covered by spores and protists. e, f) Protists, most likely belonging to the Bacillariophyceae class. g) Bacteria and bacterial filaments. h, i) Different bacterial individuals showing great varieties of shapes and morphological features.[9]
The rhizosphere comprises the 1–10 mm zone of soil immediately surrounding the roots that is under the influence of the plant through its deposition of root exudates, mucilage and dead plant cells.[10] A diverse array of organisms specialize in living in the rhizosphere, including bacteria, fungi, oomycetes, nematodes, algae, protozoa, viruses, and archaea.[11] The most frequently studied beneficial rhizosphere organisms are mycorrhizae, rhizobium bacteria, plant growth promoting rhizobacteria (PGPR), and biocontrol microbes. Gans and Wolinsky projected that one gram of soil could harbor more than a million distinct bacterial genomes.[12] İnceoğlu and Al-Soud reported 55,121 OTUs (operational taxonomic units) from the potato rhizosphere.[13] Among the prokaryotes in the rhizosphere, the most frequent bacteria are within the Acidobacteria, Proteobacteria, Planctomycetes, Actinobacteria, Bacteroidetes, and Firmicutes.[14][15] In some studies, no significant differences were reported in the microbial community composition between the bulk soil (soil not attached to the plant root) and rhizosphere soil.[16][17] Certain bacterial groups (e. g. Actinobacteria, Xanthomonadaceae) are less abundant in the rhizosphere than in nearby bulk soil .[14][1]
Mycorrhizal fungi are abundant members of the rhizosphere community, and have been found in over 200,000 plant species, and are estimated to associate with over 80% of all plants.[18] These mycorrhizae–root associations play profound roles in land ecosystems by regulating nutrient and carbon cycles. Mycorrhizae are integral to plant health because they provide up to 80 % of N and P requirements. In return, the fungi obtain carbohydrates and lipids from host plants.[19] Recent studies of arbuscular mycorrhizal fungi using sequencing technologies show greater between-species and within-species diversity than previously known.[20][1]
Phyllosphere microbiome

The aerial surface of a plant (stem, leaf, flower, fruit) is called the phyllosphere and is considered comparatively nutrient poor when compared to the rhizosphere and endosphere. The environment in the phyllosphere is more dynamic than the rhizosphere and endosphere environments. Microbial colonizers are subjected to diurnal and seasonal fluctuations of heat, moisture, and radiation. In addition, these environmental elements affect plant physiology (such as photosynthesis, respiration, water uptake etc.) and indirectly influence microbiome composition.[1] Rain and wind also cause temporal variation to the phyllosphere microbiome.[22] Overall, there remains high species richness in phyllosphere communities. Fungal communities are highly variable in the phyllosphere of temperate regions and are more diverse than in tropical regions.[23] There can be up to 107 microbes per cm2 present on leaf surfaces of plants, and thus the bacterial population of the phyllosphere on a global scale is estimated to be 1026 cells.[24] The population size of the fungal phyllosphere is likely to be smaller [25]. Phyllosphere microbes from different plants appear to be somewhat similar at high levels of taxa, but at the lower levels taxa there remain significant differences. This indicates that microorganisms may need finely tuned metabolic adjustment to survive in phyllosphere environment.[23] Proteobacteria seems to be the dominant colonizers, with Bacteroidetes and Actinobacteria also predominant in phyllospheres.[25] Although there are similarities between the rhizosphere and soil microbial communities, very low similarity has been reported between phyllosphere communities and those in open air.[26][1]
Endosphere microbiome
Some microorganisms, such as endophytes, penetrate and occupy the plant internal tissues, forming the endospheric microbiome. The AM and other endophytic fungi are the dominant colonizers of the endosphere.[27] Bacteria, and to some degree Archaea, are important members of endosphere communities. Some of these endophytic microbes interact with their host and provide obvious benefits to plants.[14][28][29] Unlike the rhizosphere and the rhizoplane, the endospheres harbor highly specific microbial communities. The root endophytic community can be very distinct from that of the adjacent soil community. In general, diversity of the endophytic community is lower than the diversity of the microbial community outside the plant.[17] The identity and diversity of the endophytic microbiome of above-and below-ground tissues may also differ within the plant.[27][1]
References
- ^ a b c d e f g h i Dastogeer, K.M., Tumpa, F.H., Sultana, A., Akter, M.A. and Chakraborty, A. (2020) "Plant microbiome–an account of the factors that shape community composition and diversity". Current Plant Biology: 100161. doi:10.1016/j.cpb.2020.100161.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Compant, S., Samad, A., Faist, H. and Sessitsch, A. (2019) "A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application". Journal of advanced research, 19: 29_37.doi:10.1016/j.jare.2019.03.004.
- ^ Shelake, R.M., Pramanik, D. and Kim, J.Y. (2019) "Exploration of plant-microbe interactions for sustainable agriculture in CRISPR era". Microorganisms, 7(8): 269. doi:10.3390/microorganisms7080269.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Koide, Roger T.; Mosse, Barbara (2004). "A history of research on arbuscular mycorrhiza". Mycorrhiza. 14 (3): 145–163. doi:10.1007/s00572-004-0307-4. PMID 15088135. S2CID 1809402.
- ^ Heckman, D. S. (2001). "Molecular Evidence for the Early Colonization of Land by Fungi and Plants". Science. 293 (5532): 1129–1133. doi:10.1126/science.1061457. PMID 11498589. S2CID 10127810.
- ^ Berendsen, Roeland L.; Pieterse, Corné M.J.; Bakker, Peter A.H.M. (2012). "The rhizosphere microbiome and plant health". Trends in Plant Science. 17 (8): 478–486. doi:10.1016/j.tplants.2012.04.001. hdl:1874/255269. PMID 22564542.
- ^ a b Bulgarelli, Davide; Schlaeppi, Klaus; Spaepen, Stijn; Van Themaat, Emiel Ver Loren; Schulze-Lefert, Paul (2013). "Structure and Functions of the Bacterial Microbiota of Plants". Annual Review of Plant Biology. 64: 807–838. doi:10.1146/annurev-arplant-050312-120106. PMID 23373698.
- ^ Turner, Thomas R.; James, Euan K.; Poole, Philip S. (2013). "The plant microbiome". Genome Biology. 14 (6): 209. doi:10.1186/gb-2013-14-6-209. PMC 3706808. PMID 23805896.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hassani, M.A., Durán, P. and Hacquard, S. (2018) "Microbial interactions within the plant holobiont". Microbiome, 6(1): 58. doi:10.1186/s40168-018-0445-0.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Hinsinger, Philippe; Bengough, A. Glyn; Vetterlein, Doris; Young, Iain M. (2009). "Rhizosphere: Biophysics, biogeochemistry and ecological relevance". Plant and Soil. 321 (1–2): 117–152. doi:10.1007/s11104-008-9885-9. S2CID 8997382.
- ^ Bonkowski, Michael; Villenave, Cécile; Griffiths, Bryan (2009). "Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots". Plant and Soil. 321 (1–2): 213–233. doi:10.1007/s11104-009-0013-2. S2CID 35701713.
- ^ Gans, J.; Wolinsky, M.; Dunbar, J. (2005). "Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil". Science. 309 (5739): 1387–1390. doi:10.1126/science.1112665. PMID 16123304. S2CID 130269020.
- ^ i̇Nceoğlu, Özgül; Al-Soud, Waleed Abu; Salles, Joana Falcão; Semenov, Alexander V.; Van Elsas, Jan Dirk (2011). "Comparative Analysis of Bacterial Communities in a Potato Field as Determined by Pyrosequencing". PLOS ONE. 6 (8): e23321. doi:10.1371/journal.pone.0023321. PMC 3158761. PMID 21886785.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Bulgarelli, Davide; Rott, Matthias; Schlaeppi, Klaus; Ver Loren Van Themaat, Emiel; Ahmadinejad, Nahal; Assenza, Federica; Rauf, Philipp; Huettel, Bruno; Reinhardt, Richard; Schmelzer, Elmon; Peplies, Joerg; Gloeckner, Frank Oliver; Amann, Rudolf; Eickhorst, Thilo; Schulze-Lefert, Paul (2012). "Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota". Nature. 488 (7409): 91–95. doi:10.1038/nature11336. PMID 22859207. S2CID 4393146.
- ^ Uroz, Stéphane; Buée, Marc; Murat, Claude; Frey-Klett, Pascale; Martin, Francis (2010). "Pyrosequencing reveals a contrasted bacterial diversity between oak rhizosphere and surrounding soil". Environmental Microbiology Reports. 2 (2): 281–288. doi:10.1111/j.1758-2229.2009.00117.x. PMID 23766079.
- ^ Lundberg, Derek S.; Lebeis, Sarah L.; Paredes, Sur Herrera; Yourstone, Scott; Gehring, Jase; Malfatti, Stephanie; Tremblay, Julien; Engelbrektson, Anna; Kunin, Victor; Rio, Tijana Glavina del; Edgar, Robert C.; Eickhorst, Thilo; Ley, Ruth E.; Hugenholtz, Philip; Tringe, Susannah Green; Dangl, Jeffery L. (2012). "Defining the core Arabidopsis thaliana root microbiome". Nature. 488 (7409): 86–90. doi:10.1038/nature11237. PMC 4074413. PMID 22859206.
- ^ a b Schlaeppi, K.; Dombrowski, N.; Oter, R. G.; Ver Loren Van Themaat, E.; Schulze-Lefert, P. (2014). "Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives". Proceedings of the National Academy of Sciences. 111 (2): 585–592. doi:10.1073/pnas.1321597111. PMID 24379374. S2CID 13806811.
- ^ Van Der Heijden, Marcel G. A.; Martin, Francis M.; Selosse, Marc-André; Sanders, Ian R. (2015). "Mycorrhizal ecology and evolution: The past, the present, and the future". New Phytologist. 205 (4): 1406–1423. doi:10.1111/nph.13288. PMID 25639293.
- ^ Rich, Mélanie K.; Nouri, Eva; Courty, Pierre-Emmanuel; Reinhardt, Didier (2017). "Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter?" (PDF). Trends in Plant Science. 22 (8): 652–660. doi:10.1016/j.tplants.2017.05.008. PMID 28622919.
- ^ Lee, Eun-Hwa; Eo, Ju-Kyeong; Ka, Kang-Hyeon; Eom, Ahn-Heum (2013). "Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems". Mycobiology. 41 (3): 121–125. doi:10.5941/MYCO.2013.41.3.121. PMC 3817225. PMID 24198665.
- ^ He, Sheng Yang (2020) When plants and their microbes are not in sync, the results can be disastrous The Conversation, 28 August 2020.
- ^ Lindow, Steven E. (1996). "Role of Immigration and Other Processes in Determining Epiphytic Bacterial Populations". Aerial Plant Surface Microbiology. pp. 155–168. doi:10.1007/978-0-585-34164-4_10. ISBN 978-0-306-45382-3.
- ^ a b Finkel, Omri M.; Burch, Adrien Y.; Lindow, Steven E.; Post, Anton F.; Belkin, Shimshon (2011). "Geographical Location Determines the Population Structure in Phyllosphere Microbial Communities of a Salt-Excreting Desert Tree". Applied and Environmental Microbiology. 77 (21): 7647–7655. doi:10.1128/AEM.05565-11. PMC 3209174. PMID 21926212.
- ^ Vorholt, Julia A. (2012). "Microbial life in the phyllosphere". Nature Reviews Microbiology. 10 (12): 828–840. doi:10.1038/nrmicro2910. PMID 23154261. S2CID 10447146.
- ^ Bodenhausen, Natacha; Horton, Matthew W.; Bergelson, Joy (2013). "Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis thaliana". PLOS ONE. 8 (2): e56329. doi:10.1371/journal.pone.0056329. PMC 3574144. PMID 23457551.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Vokou, Despoina; Vareli, Katerina; Zarali, Ekaterini; Karamanoli, Katerina; Constantinidou, Helen-Isis A.; Monokrousos, Nikolaos; Halley, John M.; Sainis, Ioannis (2012). "Exploring Biodiversity in the Bacterial Community of the Mediterranean Phyllosphere and its Relationship with Airborne Bacteria". Microbial Ecology. 64 (3): 714–724. doi:10.1007/s00248-012-0053-7. PMID 22544345. S2CID 17291303.
- ^ a b Vokou, Despoina; Vareli, Katerina; Zarali, Ekaterini; Karamanoli, Katerina; Constantinidou, Helen-Isis A.; Monokrousos, Nikolaos; Halley, John M.; Sainis, Ioannis (2012). "Exploring Biodiversity in the Bacterial Community of the Mediterranean Phyllosphere and its Relationship with Airborne Bacteria". Microbial Ecology. 64 (3): 714–724. doi:10.1007/s00248-012-0053-7. PMID 22544345. S2CID 17291303.
- ^ Dastogeer, Khondoker M.G.; Li, Hua; Sivasithamparam, Krishnapillai; Jones, Michael G.K.; Du, Xin; Ren, Yonglin; Wylie, Stephen J. (2017). "Metabolic responses of endophytic Nicotiana benthamiana plants experiencing water stress". Environmental and Experimental Botany. 143: 59–71. doi:10.1016/j.envexpbot.2017.08.008.
- ^ Rodriguez, R. J.; White Jr, J. F.; Arnold, A. E.; Redman, R. S. (2009). "Fungal endophytes: Diversity and functional roles". New Phytologist. 182 (2): 314–330. doi:10.1111/j.1469-8137.2009.02773.x. PMID 19236579.
Reference books
- Saleem M (2015) Microbiome Community Ecology: Fundamentals and Applications Springer. ISBN 9783319116655.
- Kumar V, Prasad R, Kumar M and Choudhary DK (2019) Microbiome in Plant Health and Disease: Challenges and Opportunities Springer. ISBN 9789811384950.
- Kumar, Vivek; Prasad, Ram; Kumar, Manoj; Choudhary, Devendra K. (10 August 2019). Microbiome in Plant Health and Disease: Challenges and Opportunities. ISBN 9789811384950.
- Grube, Martin; Schloter, Michael; Smalla, Kornelia; Berg, Gabriele (22 January 2015). The plant microbiome and its importance for plant and human health. ISBN 9782889193783. PMID 25278934.
- Egamberdieva, Dilfuza; Ahmad, Parvaiz (6 February 2018). Plant Microbiome: Stress Response. ISBN 9789811055140.
- Castiglione, Stefano; Cicatelli, Angela; Ferrol, Nuria; Rozpadek, Piotr (22 August 2019). Effects of Plant-Microbiome Interactions on Phyto- and Bio-Remediation Capacity. ISBN 9782889459322.
- Castiglione, Stefano; Cicatelli, Angela; Ferrol, Nuria; Rozpadek, Piotr (22 August 2019). Effects of Plant-Microbiome Interactions on Phyto- and Bio-Remediation Capacity. ISBN 9782889459322.
- Carvalhais, Lilia C.; Dennis, Paul G. (30 December 2020). The Plant Microbiome: Methods and Protocols. ISBN 9781071610398.
- Kumar, Vivek; Prasad, Ram; Kumar, Manoj; Choudhary, Devendra K. (10 August 2019). Microbiome in Plant Health and Disease: Challenges and Opportunities. ISBN 9789811384950.
- Solanki, Manoj Kumar; Kashyap, Prem Lal; Ansari, Rizwan Ali; Kumari, Baby (28 August 2020). Microbiomes and Plant Health: Panoply and Their Applications. ISBN 9780128226018.
- Yadav, Ajar Nath (2020). Advances in Plant Microbiome and Sustainable Agriculture: Diversity and Biotechnological Applications. ISBN 9789811532085.
- Varma, Ajit. Plant Microbiome Paradigm. ISBN 9783030503956.
- Doty, Sharon Lafferty (21 September 2017). Functional Importance of the Plant Microbiome: Implications for Agriculture, Forestry and Bioenergy. ISBN 9783319658971.
- Antwis, Rachael E.; Harrison, Xavier A.; Cox, Michael J. (12 March 2020). Microbiomes of Soils, Plants and Animals: An Integrated Approach. ISBN 9781108473712.
- Varma, Ajit; Tripathi, Swati; Prasad, Ram (20 October 2020). Plant Microbiome Paradigm. ISBN 9783030503949.
- Rosenberg, Eugene; Zilber-Rosenberg, Ilana (31 January 2014). The Hologenome Concept: Human, Animal and Plant Microbiota. ISBN 9783319042411.
- Yadav, Ajar Nath; Singh, Joginder; Rastegari, Ali Asghar; Yadav, Neelam (6 March 2020). Plant Microbiomes for Sustainable Agriculture. ISBN 9783030384531.

