Aromatic sulfonation
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution.[1]

Mechanism
Sulfur trioxide is the electrophile in this electrophilic aromatic substitution generated from concentrated sulfuric acid (or fuming sulfuric acid) when heated. With benzene as substrate the reaction product is benzenesulfonic acid.
In contrast to aromatic nitration and other electrophilic aromatic substitutions this reaction is reversible. Sulfonation takes place in concentrated acidic conditions and desulfonation is the mode of action in a dilute hot aqueous acid. It is very useful in protecting the aromatic system because of this reversibility.
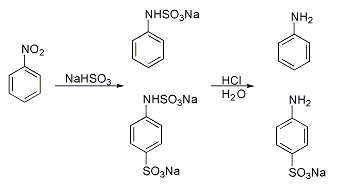
Piria reaction
A classic named reaction is the Piria reaction (R. Piria, 1851) in which nitrobenzene is reacted with a metal bisulfite forming an aminosulfonic acid as a result of combined nitro group reduction and sulfonation.[2][3]
Tyrer sulfonation process
In the Tyrer sulfonation process (1917),[4] at some time of technological importance, benzene vapor is led through a vessel containing 90% sulfuric acid the temperature of which is increased from 100 to 180°C. Water and benzene are continuously removed in a condenser and the benzene layer fed back to the vessel. In this way a 80% yield is obtained.

Application
Aromatic sulfonic acids are intermediates in the preparation of dyes and many pharmaceuticals. Sulfonation of aniline produces p-aminobenzenesulfonic acid or sulfanilic acid which is a zwitterion with an unusual high melting point. The amide of this compound and related compounds form a large group of sulfa drugs.
Sulfonation of polystyrene can be used to prepare sodium polystyrene sulfonate.
See also
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595.
- ^ Piria, Ann. 78 31 (1851).
- ^ THE PIRIA REACTION. I. THE OVER-ALL REACTION W. H. Hunter, Murray M. Sprung J. Am. Chem. Soc., 1931, 53 (4), pp 1432–1443 doi:10.1021/ja01355a037.
- ^ U.S. patent 1,210,725
- ^ Siegfried Hauptmann: Organische Chemie, 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, ISBN 3-342-00280-8.

