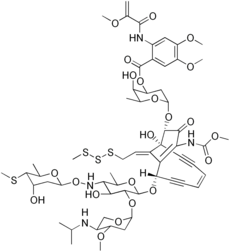
Esperamicin
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C59H80N4O22S4 | |
| Molar mass | 1325.54 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The esperamicins are chromoprotein enediyne antitumor antibiotics of bacterial origin. Esperamicin A1 is the most well studied compound in this class. Esperamcin A1 and the related enediyne calicheamicin are the two most potent antitumor agents known.[1] The esperamicins are extremely toxic DNA splicing compounds.
Oxygen and active oxygen-radical scavengers have no significant influence upon DNA strand breakage by esperamicin, but the cleavage of DNA by esperamicin is greatly accelerated in the presence of thiol compounds. The preferential cutting sites of esperamicin are at thymidylate residues, and the frequency of nucleobase attacked (T greater than C greater than A greater than G) is different from that of calicheamicin (C much greater than T greater than A = G), neocarzinostatin (T greater than A greater than C greater than G), or bleomycin (C greater than T greater than A greater than G).[2]
References
- ^ Calicheamicin and Esperamicin are the two most potent antitumor agents known to man, Univ Of Georgia Chem 4500 Archived September 21, 2008, at the Wayback Machine
- ^ Nucleotide-specific cleavage and minor-groove interaction of DNA with esperamicin antitumor antibiotics
