Immunoreceptor tyrosine-based activation motif
An immunoreceptor tyrosine-based activation motif (ITAM) (in the antagonistic case ITIM, I for inhibition) is a conserved sequence of four amino acids that is repeated twice in the cytoplasmic tails of certain cell surface proteins of the immune system.[1]
Structure
The motif contains a tyrosine separated from a leucine or isoleucine by any two other amino acids, giving the signature YxxL/I.[1] Two of these signatures are typically separated by between 6 and 8 amino acids in the tail of the molecule (YxxL/Ix(6-8)YxxL/I).
Function
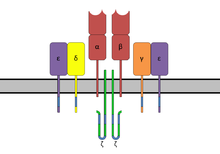
ITAMs are important for signal transduction in immune cells. Hence, they are found in the tails of important cell signaling molecules such as the CD3 and ζ-chains of the T cell receptor complex,[1] the CD79-alpha and -beta chains of the B cell receptor complex, and certain Fc receptors. The tyrosine residues within these motifs become phosphorylated following interaction of the receptor molecules with their ligands and form docking sites for other proteins involved in the signaling pathways of the cell.
References
- ^ a b c Abbas, Abul K; Lichtman, Andrew H. (2009), Basic Immunology: Functions and Disorders of the Immune System (3 ed.), Philadelphia, PA: Saunders, ISBN 978-1-4160-4688-2
