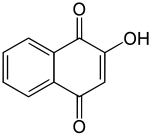
Lawsone
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxynaphthalene-1,4-dione | |
| Other names
2-Hydroxy-1,4-naphthoquinone
Hennotannic acid Natural Orange 6 C.I. 75480 | |
| Identifiers | |
3D model (JSmol)
|
|
| 1565260 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.361 |
| EC Number |
|
| 4828 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H6O3 | |
| Molar mass | 174.15 g/mol |
| Appearance | Yellow prisms |
| Melting point | 195 to 196 °C (383 to 385 °F; 468 to 469 K) (decomposition) |
| almost insoluble [3] | |
| Hazards[4] | |
| GHS labelling: | |
 
| |
| Warning | |
| H315, H319, H335 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
100 mg/kg |
| Related compounds | |
Related naphthoquinones
|
Juglone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant (Lawsonia inermis), for which it is named, as well as in the flower of water hyacinth (Eichhornia crassipes).[5] Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5,000 years. Lawsone reacts chemically with the protein keratin in skin and hair via Michael addition, resulting in a strong permanent stain that lasts until the skin or hair is shed. The darker colored ink is due to more lawsone-keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades.[6] Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning and sunscreens. Lawsone is a 1,4-naphthoquinone derivative.
Lawsone isolation from Lawsonia Inermis can be difficult due to its easily biodegradable nature. Isolation involves four steps:
- extraction with an extraction solution, usually NaOH
- column filtration using a macroporous adsorption resin
- a rinse with ethanol to remove impurities, and finally
- freeze the product to isolate the lawsone powder, usually a yellow colored dust.[7]
During the rinse, the lawsone will be the bottom as it has such a high density and the chlorophyll molecules will all be on the top of the mixture.[8]
Lawsone is hypothesized to undergo a reaction similar to Strecker synthesis in reactions with amino acids.[9] Recent research has been conducted on lawsone's potential applications in the forensic science field. Since lawsone shows many similarities with ninhydrin, the current reagent for latent fingerprint development, studies have been conducted to see if lawsone can be used in this field. As of now the research is inconclusive, but optimistic. Lawsone non-specifically targets primary amino acids, and displays photoluminescence with forensic light sources.[9] It has a characteristic purple/brown coloration as opposed to the purple/blue associated with ninhydrin.[10] Lawsone shows promise as a reagent for fingerprint detection because of its photoluminescence maximized at 640 nm, which is high enough that it avoids background interference common for ninhydrin.[11]
Related compounds
The naphthoquinones lawsone methyl ether and methylene-3,3'-bilawsone are some of the active compounds in Impatiens balsamina leaves.[12] Juglone is a structural isomer used as a brown dye.
References
- ^ Merck Index, 12th Edition, 5406.
- ^ MSDS Archived 2005-12-17 at the Wayback Machine at Physical & Theoretical Chemistry Laboratory, University of Oxford
- ^ http://msds.chem.ox.ac.uk/HY/2-hydroxy-1,4-naphthaquinone.html [dead link]
- ^ "2-Hydroxy-1,4-naphthoquinone". pubchem.ncbi.nlm.nih.gov.
- ^ Dweck, A. C. (2002). "Natural ingredients for colouring and styling". International Journal of Cosmetic Science. 24 (5): 287–302. doi:10.1046/j.1467-2494.2002.00148.x. PMID 18498522.
- ^ Jordão, A.; Vargas, M.; Pinto, A.; da Silva, F.; Ferreira, V. Lawsone in organic synthesis. RSC Adv. 2015, 5, 67909-67943.
- ^ Shuang, S.; Lei, Q.; Ting, Y.; Qifu, Y. Method for preparing lawsone from lawsonia inermis China Patent CN 103848732A, June 11, 2014.
- ^ Gallo, F.; Multari, G.; Giambenedetti, M.; Federici, E. Chemical fingerprinting of Lawsonia inermis L. using HPLC, HPTLC, and densitometry. Phytochem. Anal. 2008, 19, 550-559.
- ^ a b Jelly, R.; Lewis, S. W.;Lennard, C.; Lim, K. F.; Almog, J. Lawsone: a novel reagent for the detection of latent fingermarks on paper surfaces. Chem.Commun. 2008, 3513-3515
- ^ Jelly, R.; Lewis, S. W.; Lennard, C.; Lim, K. F.; Almog, J. Lawsone: a novel reagent for the detection of latent fingermarks on paper surfaces. Chem. Commun. 2008, 3513-3515.
- ^ Thomas, P.; Farrugia, K. An investigation into the enhancement of fingermarks in blood on paper with genipin and lawsone. Sci. Justice 2013, 53, 315-320.
- ^ Sakunphueak, A.; Panichayupakaranant, P. (2010). "Simultaneous determination of three naphthoquinones in the leaves of Impatiens balsamina L. by reversed‐phase high‐performance liquid chromatography". Phytochemical Analysis. 21 (5): 444–50. doi:10.1002/pca.1216. PMID 20931623.
