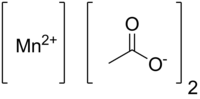
Manganese(II) acetate
| |
| Names | |
|---|---|
| IUPAC name
Manganese(II) acetate
| |
| Other names
Manganese diacetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.010.305 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mn(CH3COO)2 (anhydrous) Mn(CH3COO)2·4H2O (tetrahydrate) | |
| Molar mass | 173.027 g/mol (anhydrous) 245.087 g/mol (tetrahydrate) |
| Appearance | red crystals (anhydrous) red monoclinic crystals (tetrahydrate) |
| Density | 1.74 g/cm3 (anhydrous) 1.59 g/cm3 (tetrahydrate) |
| Melting point | 210°C (anhydrous) 80°C (tetrahydrate) |
| Solubility | soluble in water, methanol, acetic acid (anhydrous) soluble in water, ethanol (tetrahydrate) |
| Related compounds | |
Other anions
|
Manganese(II) fluoride Manganese(II) chloride Manganese(II) bromide |
Other cations
|
Zinc acetate Mercury(II) acetate Silver acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Manganese(II) acetate is the chemical compound with the formula Mn(CH3COO)2. It is used as a desiccant, a catalyst, and as fertilizer.[2]
Reactions
Manganese(II) acetate can be formed by reacting acetic acid with either manganese(II,III) oxide or manganese(II) carbonate[2][3]:
- Mn3O4 + 2CH3COOH → Mn(CH3COO)2 + Mn2O3 + H2O
If manganese(II,III) oxide is used, manganese(III) oxide is produced as a byproduct.
If the anhydrous form needs to be produced, manganese(II) nitrate can be reacted with acetic anhydride.[2]
References
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–354, 4–68, ISBN 0-8493-0594-2
- ^ a b c Thomas Scott; Mary Eagleson (1994), Concise encyclopedia chemistry, Walter de Gruyter, p. 620, ISBN 3-11-011451-8, retrieved 2009-07-20
- ^ Patnaik, Pradyot (2003), Handbook of Inorganic Chemical Compounds, McGraw-Hill Professional, pp. 81–82, ISBN 0-07-049439-8, retrieved 2009-07-20
