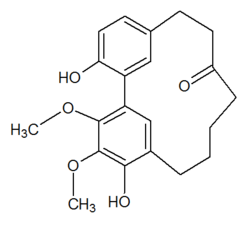
Myricanone
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H24O5 | |
| Molar mass | 356.41 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Myricanone is a cyclic diarylheptanoid isolated from the bark of Myrica rubra (Myricaceae).[1]
References
- ^ Akazawa, H; Fujita, Y; Banno, N; Watanabe, K; Kimura, Y; Manosroi, A; Manosroi, J; Akihisa, T (2010). "Three new cyclic diarylheptanoids and other phenolic compounds from the bark of Myrica rubra and their melanogenesis inhibitory and radical scavenging activities". Journal of Oleo Science. 59 (4): 213–221. doi:10.5650/jos.59.213. PMID 20299768.
