Ocimene
| |
| Names | |
|---|---|
| IUPAC names
α: cis-3,7-dimethyl-1,3,7-octatriene
β: trans-3,7-dimethyl-1,3,6-octatriene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.205 |
PubChem CID
|
|
| |
| |
| Properties | |
| C10H16[1] | |
| Molar mass | 136.24 g/mol |
| Density | 0.800 g/cm3 |
| Melting point | 50 °C |
| Boiling point | mix of isomers: 100 °C at 70 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
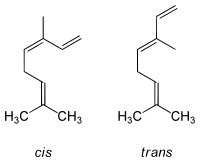
Ocimene refers to several isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is cis-3,7-dimethyl-1,3,7-octatriene. β-Ocimene is trans-3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, cis and trans, with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery. Like the related acyclic terpene myrcene, ocimenes are unstable in air.[2] Like other terpenes, the ocimens are nearly insoluble in water, but soluble in common organic solvents.
References
- ^ "CID 5281553 -- PubChem Compound Summary". Retrieved 2008-02-17.
- ^ Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_141
