Osmium(IV) chloride
| |
| Names | |
|---|---|
| IUPAC name
Osmium(IV) chloride
| |
| Other names
Osmium chloride, osmium tetrachloride
| |
| Identifiers | |
| ECHA InfoCard | 100.151.226 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| OsCl4 | |
| Molar mass | 332.041 g/mol |
| Appearance | red-black orthorhombic crystals |
| Density | 4.38 g/cm³ |
| Melting point | decomposes at 323°C |
| reacts with water | |
| Solubility | soluble in organic solvents[1] |
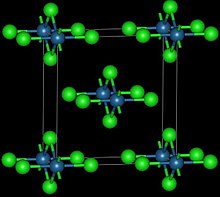
| Structure | |
| Orthorhombic, oS10 | |
| Cmmm, No. 65 | |
| Related compounds | |
Other anions
|
Osmium(IV) oxide |
Other cations
|
Iron(III) chloride Ruthenium(III) chloride Osmium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Osmium(IV) chloride or osmium tetrachloride is the chemical compound composed of osmium and chlorine with the formula OsCl4. It forms reddish-black orthorhombic crystals[1], in which osmium atoms are octahedrally coordinated with sharing of opposite edges of the OsCl6 octahedra to form a chain.[2] It is an oxidizing agent.
Reactions
Osmium(IV) chloride is formed by dissolving Osmium(VIII) oxide in concentrated hydrochloric acid [3]
- OsO4 + 8 HCl → OsCl4 + 4 H2O + 2 Cl2
or by heating elemental osmium with chlorine at high pressures[4]:
- Os + 2Cl2 → OsCl4
Osmium(VIII) oxide can be formed by the oxidation of osmium(IV) chloride with sodium hypochlorite[5].
- OsCl4 + 4 NaClO → OsO4 + 4 NaCl + 2 Cl2
References
- ^ a b Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 479, ISBN 0849386713, retrieved 2008-06-27
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Thorpe, Thomas Edward (2004), A Dictionary of Applied Chemistry, vol. 4, London: Longmans, Green, and Co., p. 727, retrieved 2008-06-27
- ^ "Chemistry: Periodic Table: Osmium: chemical reaction data". WebElements. Retrieved 2008-06-27.
- ^ Patnaik, Pradyot (2004), Handbook of Inorganic Chemicals, Amsterdam: McGraw-Hill Professional, p. 672, ISBN 0070494398, retrieved 2008-06-27
