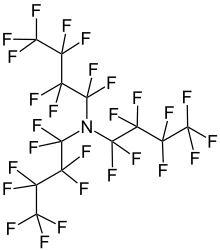
Perfluorotributylamine
| |
| Names | |
|---|---|
| IUPAC name
1,1,2,2,3,3,4,4,4-nonafluoro-N,N-bis(1,1,2,2,3,3,4,4,4-nonafluorobutyl)butan-1-amine
| |
| Other names
Fluorinert
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | PFTBA |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.659 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12F27N | |
| Molar mass | 671.096 g·mol−1 |
| Density | 1.884 g/mL |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 178 °C (352 °F; 451 K) |
| Insoluble | |
| Solubility in methanol and isopropyl alcohol | Insoluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perfluorotributylamine (PFTBA), also referred to as FC43, is a liquid with a molecular structure consisting of three butyl moieties connected to an amine center, in which the hydrogen atoms have all been replaced with fluorine. The compound is produced by the electronic industry, along with other perfluoroalkyl amines.[1] It is used as an ingredient in Fluosol and in some Fluorinert coolant liquids.[2]
Environmental Impact
In 2013, this liquid was shown by researchers at the University of Toronto to be a greenhouse gas, with warming properties more than 7,000 times that of carbon dioxide over a 100 year period,[3][4] and that, as such, it is the most potent greenhouse gas ever discovered.[5] Its concentration in the atmosphere is approximately 0.18 parts per trillion. The researchers also reported that the gas can persist in the atmosphere for up to 500 years.
-
Global warming potential of greenhouse gases and PFTBA
Uses
Industrial Uses
The compound is used in larger quantities in several electronic applications, such as liquid burn-in, testing, environmental stress screening and vapor phase soldering processes as well as an indicator fluid in leak testing and as a heat transfer fluid for thermal shock testing.
Mass Spectrometer Tuning Compound
The compound is commonly used in small quantities (1 ml) as a calibration chemical[6] in gas chromatography when the analytical technique uses mass spectrometry as a detector to identify and quantify chemical compounds in gases or liquids. When undergoing ionization in the mass spectrometer, the compound decomposes in a repeatable pattern to form fragments of specific masses, which can be used to tune the mass response and accuracy of the mass spectrometer. Most commonly used ions are those with approximate mass of 69, 131, 219 and 502 atomic mass units.
See also
References
- ^ Upton, John (10 December 2013). "Meet perfluorotributylamine, the world's worst greenhouse gas". Grist. Retrieved 11 December 2013.
- ^ Garrelts, J. C. (1990). "Fluosol: An oxygen-delivery fluid for use in percutaneous transluminal coronary angioplasty". DICP : the annals of pharmacotherapy. 24 (11): 1105–1112. PMID 2275237.
- ^ Hong, A. C.; Young, C. J.; Hurley, M. D.; Wallington, T. J.; Mabury, S. A. (2013). "Perfluorotributylamine: A novel long-lived greenhouse gas". Geophysical Research Letters: n/a. doi:10.1002/2013GL058010.
- ^ Goldenberg, Suzanne (10 December 2013). "Newly discovered greenhouse gas '7,000 times more powerful than CO2'". The Guardian. Retrieved 11 December 2013.
- ^ Goldenberg, Suzanne (11 December 2013). "Newly Discovered Greenhouse Gas "7,000 Times More Powerful than CO2"". Mother Jones. Retrieved 12 December 2013.
- ^ Dunnivant, Frank and Ginsbach, Jake. "GAS CHROMATOGRAPHY, LIQUID CHROMATOGRAPHY, CAPILLARY ELECTROPHORESIS - MASS SPECTROMETRY A BASIC INTRODUCTION", Chapter 7, ISBN 978-0-9882761-0-9, [1]., Nov. 2012.

