Potassium hexanitritocobaltate(III)
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium hexanitritocobaltate(III)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.034.018 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| K3[Co(NO2)6] (anhydrous) K3[Co(NO2)6]·1.5H2O (sesquihydrate) | |
| Molar mass | 452.26 g/mol (anhydrous) 479.284 g/mol (sesquihydrate) |
| Appearance | yellow cubic crystals (sesquihydrate) |
| Density | 2.6 g/cm3 (sesquihydrate) |
| slightly soluble in water (sesquihydrate) | |
| Solubility | reacts with acids, insoluble in ethanol (sesquihydrate)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
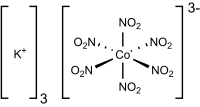
Potassium cobaltinitrite, IUPAC name potassium hexanitritocobaltate(III), is a coordination compound with the formula K3[Co(NO2)6]. The anion of this yellow-coloured salt consists of a cobalt(III) center bound to six nitrito ligands. It is insoluble in water and is precipitated as yellow solids.
It was first made in 1848 by Nikolaus Wolfgang Fischer in Breslau,[2] and it is used as a yellow pigment called Aureolin.[3][4]
See also
References
- ^
Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–54. ISBN 0-8493-0594-2Template:Inconsistent citations
{{cite book}}: CS1 maint: postscript (link) - ^ Fischer, N. W. (1848). "Ueber die salpetrichtsauren Salze". Annalen der Physik und Chemie. 150 (5): 115–125. Bibcode:1848AnP...150..115F. doi:10.1002/andp.18491500512.
- ^ Gates, G. (1995). "A Note on the Artists' Pigment Aureolin". Studies in Conservation. 40 (3): 201–206. doi:10.2307/1506479. JSTOR 1506479.
- ^ Gettens, Rutherford John; Stout, George Leslie (1966). Painting materials: A short encyclopaedia. pp. 109–110. ISBN 978-0-486-21597-6.
