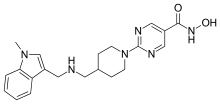
Quisinostat
 | |
| Clinical data | |
|---|---|
| Other names | JNJ-26481585 |
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H26N6O2 |
| Molar mass | 394.470 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Quisinostat (USAN;[1] development code JNJ-26481585) is an experimental drug candidate for the treatment of cancer. It is a "second generation" histone deacetylase inhibitor with antineoplastic activity.[2][3][4]
References
- ^ "Quisinostat" (PDF). American Medical Association.
- ^ Tong, Wei-Gang; Wei, Yue; Stevenson, William; Kuang, Shao-Qing; Fang, Zhihong; Zhang, Ming; Arts, Janine; Garcia-Manero, Guillermo (2010). "Preclinical antileukemia activity of JNJ-26481585, a potent second-generation histone deacetylase inhibitor". Leukemia Research. 34 (2): 221–8. doi:10.1016/j.leukres.2009.07.024. PMID 19682743.
- ^ Stühmer, Thorsten; Arts, Janine; Chatterjee, Manik; Borawski, Johanna; Wolff, André; King, Peter; Einsele, Hermann; Leo, Eugen; Bargou, Ralf C. (2010). "Preclinical anti-myeloma activity of the novel HDAC-inhibitor JNJ-26481585". British Journal of Haematology. 149 (4): 529–36. doi:10.1111/j.1365-2141.2010.08126.x. PMID 20331455.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ "Quisinostat". NCI Drug Dictionary. National Cancer Institute.
