Reactive oxygen species
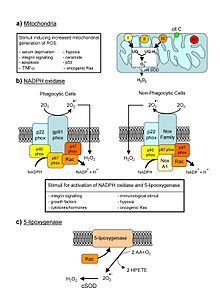
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons. ROS form as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling and homeostasis.[1] However, during times of environmental stress (e.g., UV or heat exposure), ROS levels can increase dramatically.[1] This may result in significant damage to cell structures. Cumulatively, this is known as oxidative stress. ROS are also generated by exogenous sources such as ionizing radiation.
Signaling and damaging effects
Normally, cells defend themselves against ROS damage with enzymes such as alpha-1-microglobulin, superoxide dismutases, catalases, lactoperoxidases, glutathione peroxidases and peroxiredoxins. Small molecule antioxidants such as ascorbic acid (vitamin C), tocopherol (vitamin E), uric acid, and glutathione also play important roles as cellular antioxidants. In similar manner, polyphenol antioxidants assist in preventing ROS damage by scavenging free radicals. In contrast, the antioxidant ability of the extracellular space is less - e.g., the most important plasma antioxidant in humans is uric acid.
Effects of ROS on cell metabolism are well documented in a variety of species. These include not only roles in apoptosis (programmed cell death) but also positive effects such as the induction of host defence[2][3]genes and mobilisation of ion transport systems.[citation needed] This implicates them in redox signaling, also known as "oxidative signaling". In particular, platelets involved in wound repair and blood homeostasis release ROS to recruit additional platelets to sites of injury. These also provide a link to the adaptive immune system via the recruitment of leukocytes.[citation needed]
Reactive oxygen species are implicated in cellular activity to a variety of inflammatory responses including cardiovascular disease. They may also be involved in hearing impairment via cochlear damage induced by elevated sound levels, in ototoxicity of drugs such as cisplatin, and in congenital deafness in both animals and humans.[citation needed] Redox signaling is also implicated in mediation of apoptosis or programmed cell death and ischaemic injury. Specific examples include stroke and heart attack.[citation needed]
In general, harmful effects of reactive oxygen species on the cell are most often:[4]
- damage of DNA
- oxidations of polyunsaturated fatty acids in lipids (lipid peroxidation)
- oxidations of amino acids in proteins
- oxidatively inactivate specific enzymes by oxidation of co-factors
Pathogen Response
When a plant recognizes an attacking pathogen, one of the first induced reactions is to rapidly produce superoxide or hydrogen peroxide to strengthen the cell wall. This prevents the spread of the pathogen to other parts of the plant, essentially forming a net around the pathogen to restrict movement and reproduction.
Oxidative damage
In aerobic organisms the energy needed to fuel biological functions is produced in the mitochondria via the electron transport chain. In addition to energy, reactive oxygen species (ROS) with the potential to cause cellular damage are produced. ROS can damage DNA, RNA, and proteins, which, in theory, contributes to the physiology of ageing.
ROS are produced as a normal product of cellular metabolism. In particular, one major contributor to oxidative damage is hydrogen peroxide (H2O2), which is converted from superoxide that leaks from the mitochondria. Catalase and superoxide dismutase ameliorate the damaging effects of hydrogen peroxide and superoxide by converting these compounds into oxygen and water, benign molecules. However, this conversion is not 100% efficient, and residual peroxides persist in the cell. While ROS are produced as a product of normal cellular functioning, excessive amounts can cause deleterious effects.[5] Memory capabilities decline with age, evident in human degenerative diseases such as Alzheimer’s disease, which is accompanied by an accumulation of oxidative damage. Current studies demonstrate that the accumulation of ROS can decrease an organism’s fitness because oxidative damage is a contributor to senescence. In particular, the accumulation of oxidative damage may lead to cognitive dysfunction, as demonstrated in a study in which old rats were given mitochondrial metabolites and then given cognitive tests. Results showed that the rats performed better after receiving the metabolites, suggesting that the metabolites reduced oxidative damage and improved mitochondrial function.[6] Accumulating oxidative damage can then affect the efficiency of mitochondria and further increase the rate of ROS production.[7] The accumulation of oxidative damage and its implications for aging depends on the particular tissue type where the damage is occurring. Additional experimental results suggest that oxidative damage is responsible for age-related decline in brain functioning. Older gerbils were found to have higher levels of oxidized protein in comparison to younger gerbils. Treatment of old and young mice with a spin trapping compound caused a decrease in the level of oxidized proteins in older gerbils but did not have an effect on younger gerbils. In addition, older gerbils performed cognitive tasks better during treatment but ceased functional capacity when treatment was discontinued, causing oxidized protein levels to increase. This led researchers to conclude that oxidation of cellular proteins is potentially important for brain function (Carney, 1991).
Internal production
Free radicals are mainly produced inside organelles, such as the mitochondrion, and also released toward the cytosol.[8][9] Mitochondria convert energy for the cell into a usable form, adenosine triphosphate (ATP). The process in which ATP is produced, called oxidative phosphorylation, involves the transport of protons (hydrogen ions) across the inner mitochondrial membrane by means of the electron transport chain. In the electron transport chain, electrons are passed through a series of proteins via oxidation-reduction reactions, with each acceptor protein along the chain having a greater reduction potential than the previous. The last destination for an electron along this chain is an oxygen molecule. In normal conditions, the oxygen is reduced to produce water; however, in about 0.1–2% of electrons passing through the chain (this number derives from studies in isolated mitochondria, though the exact rate in live organisms is yet to be fully agreed upon), oxygen is instead prematurely and incompletely reduced to give the superoxide radical (·O2-), most well documented for Complex I and Complex III. Superoxide is not particularly reactive by itself, but can inactivate specific enzymes or initiate lipid peroxidation in its protonated form, hydroperoxyl HO2·. The pKa of hydroperoxyl is 4.8. Thus, at physiological pH, the majority will exist as superoxide.
If too much damage is caused to its mitochondria, a cell undergoes apoptosis or programmed cell death. Bcl-2 proteins are layered on the surface of the mitochondria, detect damage, and activate a class of proteins called Bax, which punch holes in the mitochondrial membrane, causing cytochrome C to leak out. This cytochrome C binds to Apaf-1, or apoptotic protease activating factor-1, which is free-floating in the cell’s cytoplasm. Using energy from the ATPs in the mitochondrion, the Apaf-1 and cytochrome C bind together to form apoptosomes. The apoptosomes bind to and activate caspase-9, another free-floating protein. The caspase-9 then cleaves the proteins of the mitochondrial membrane, causing it to break down and start a chain reaction of protein denaturation and eventually phagocytosis of the cell.
Cause of aging
According to the Free-radical theory, oxidative damage initiated by reactive oxygen species is a major contributor to the functional decline that is characteristic of aging. While studies in invertebrate models indicate that animals genetically engineered to lack specific antioxidant enzymes (such as SOD), in general, show a shortened lifespan (as one would expect from the theory), the converse manipulation, increasing the levels of antioxidant enzymes, has yielded inconsistent effects on lifespan (though some well-performed studies in Drosophila do show that lifespan can be increased by the overexpression of MnSOD or glutathione biosynthesizing enzymes).
In mice, the story is somewhat similar. Deleting antioxidant enzymes, in general, yields shorter lifespan, though overexpression studies have not (with some recent exceptions) consistently extended lifespan.[10]
Superoxide dismutase
Superoxide dismutases (SOD) are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen. In mammals and most chordates, three forms of superoxide dismutase are present. SOD1 is located in the cytoplasm, SOD2 in the mitochondria and SOD3 is extracellular. The first is a dimer (consists of two units), while the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc, while SOD2 has manganese in its reactive centre. The genes are located on chromosomes 21, 6, and 4, respectively (21q22.1, 6q25.3 and 4p15.3-p15.1).
The SOD-catalysed dismutation of superoxide may be written with the following half-reactions :
- M(n+1)+ − SOD + O2− → Mn+ − SOD + O2
- Mn+ − SOD + O2− + 2H+ → M(n+1)+ − SOD + H2O2.
where M = Cu (n=1) ; Mn (n=2) ; Fe (n=2) ; Ni (n=2).
In this reaction the oxidation state of the metal cation oscillates between n and n+1.
Catalase, which is concentrated in peroxisomes located next to mitochondria, reacts with the hydrogen peroxide to catalyze the formation of water and oxygen. Glutathione peroxidase reduces hydrogen peroxide by transferring the energy of the reactive peroxides to a very small sulfur-containing protein called glutathione. The selenium contained in these enzymes acts as the reactive center, carrying reactive electrons from the peroxide to the glutathione. Peroxiredoxins also degrade H2O2, within the mitochondria, cytosol, and nucleus.
- 2 H2O2 → 2 H2O + O2 (catalase)
- 2GSH + H2O2 → GS–SG + 2H2O (glutathione peroxidase)
ROS-directed cancer chemotherapeutics
Recent research demonstrates that redox dysregulation originating from metabolic alterations and dependence on mitogenic and survival signaling through ROS represents a specific vulnerability of malignant cells that can be selectively targeted by pro- and antioxidant redox chemotherapeutics.[11]
See also
- Antioxidant
- Melanin
- Mitohormesis
- Oxidative stress
- Oxygen toxicity
- Polyphenol antioxidants
- Pro-oxidant
- Redox signaling
- Iodide
References
- ^ a b Devasagayam, TPA (2004). "Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects". Journal of Association of Physicians of India (JAPI). 52: 796.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Rada B, Leto TL (2008). "Oxidative innate immune defenses by Nox/Duox family NADPH oxidases" (PDF). Contrib Microbiol. Contributions to Microbiology. 15: 164–87. doi:10.1159/000136357. ISBN 978-3-8055-8548-4. PMC 2776633. PMID 18511861. — Review
- ^ Conner GE, Salathe M, Forteza R (2002). "Lactoperoxidase and Hydrogen Peroxide Metabolism in the Airway". Am J Respir Crit Care Med. 166 (12): S57–61. doi:10.1164/rccm.2206018. PMID 12471090.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brooker, Robert J. (2011). Genetics: analysis and principles (4th ed.). McGraw-Hill Science. ISBN 978-0-07-352528-0.
- ^ Patel RP, T Cornwell,Darley-Usmar VM (1999). "The biochemistry of nitric oxide and peroxynitrite: implications for mitochondrial function". In Packer L, Cadenas E (ed.). Understanding the process of aging: the roles of mitochondria, free radicals, and antioxidants. New York, N.Y: Marcel Dekker. pp. 39–56. ISBN 0-8247-1723-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN (2002). "Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid". Proc. Natl. Acad. Sci. U.S.A. 99 (4): 2356–61. doi:10.1073/pnas.261709299. PMC 122369. PMID 11854529.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stadtman ER (1992). "Protein oxidation and aging". Science. 257 (5074): 1220–4. doi:10.1126/science.1355616. PMID 1355616.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Muller, Florian (2000). "The nature and mechanism of superoxide production by the electron transport chain: Its relevance to aging". AGE. 23 (4): 227–253. doi:10.1007/s11357-000-0022-9.
- ^ Han D, Williams E, Cadenas E (2001). "Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space". Biochem. J. 353 (Pt 2): 411–6. doi:10.1042/0264-6021:3530411. PMC 1221585. PMID 11139407.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007). "Trends in oxidative aging theories". Free Radic. Biol. Med. 43 (4): 477–503. doi:10.1016/j.freeradbiomed.2007.03.034. PMID 17640558.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wondrak GT (2009). "Redox-directed cancer therapeutics: molecular mechanisms and opportunities". Antioxid. Redox Signal. 11 (12): 3013–69. doi:10.1089/ARS.2009.2541. PMC 2824519. PMID 19496700.
{{cite journal}}: Unknown parameter|month=ignored (help)
Further reading
- Sen CK (2003). "The general case for redox control of wound repair". Wound Repair Regen. 11 (6): 431–8. doi:10.1046/j.1524-475X.2003.11607.x. PMID 14617282.
- Krötz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U (2002). "NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment". Blood. 100 (3): 917–24. doi:10.1182/blood.V100.3.917. PMID 12130503.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F (1998). "Hydrogen peroxide is involved in collagen-induced platelet activation". Blood. 91 (2): 484–90. PMID 9427701.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Guzik TJ, Korbut R, Adamek-Guzik T (2003). "Nitric oxide and superoxide in inflammation and immune regulation". J. Physiol. Pharmacol. 54 (4): 469–87. PMID 14726604.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Proctor PH (1989). "Free Radicals And Human Disease, a Review". drproctor.com. Retrieved 2009-07-23.
