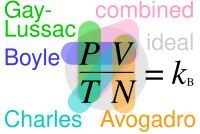
Template:Ideal gas law relationships.svg
Appearance
This is an old revision of this page, as edited by Hellacioussatyr (talk | contribs) at 17:58, 10 July 2021. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
