Autoimmune autonomic ganglionopathy: Difference between revisions
mNo edit summary |
Expanding article |
||
| Line 18: | Line 18: | ||
| specialty = <!-- from Wikidata; can be overwritten --> |
| specialty = <!-- from Wikidata; can be overwritten --> |
||
|symptoms = [[Intestinal pseudo-obstruction|Gastrointestinal dysmotility]], [[orthostatic hypotension]], and [[Adie syndrome|tonic pupils]].<ref name="Winston Vernino 2009 pp. 85–93"/> |
|symptoms = [[Intestinal pseudo-obstruction|Gastrointestinal dysmotility]], [[orthostatic hypotension]], and [[Adie syndrome|tonic pupils]].<ref name="Winston Vernino 2009 pp. 85–93"/> |
||
| complications = |
| complications = Weight loss. |
||
| onset = |
| onset = |
||
| duration = |
| duration = |
||
| Line 26: | Line 26: | ||
| risks = <!-- or |
| risks = <!-- or |
||
| risk = --> |
| risk = --> |
||
| diagnosis = |
| diagnosis = Clinical criteria and serum ganglionic neuronal nicotinic AChR antibodies. |
||
| differential = [[Paraneoplastic syndrome]], [[Guillain-Barré syndrome]], [[diabetes]], [[amyloidosis]], [[Sjögren syndrome|Sjogren's syndrome]], and [[Morvan's syndrome|Morvan syndrome]]. |
|||
| differential = |
|||
| prevention = |
| prevention = |
||
| treatment = <!-- or |
| treatment = <!-- or |
||
| Line 41: | Line 41: | ||
}} |
}} |
||
'''Autoimmune autonomic ganglionopathy''' is a type of immune-mediated autonomic failure that is associated with antibodies against the ganglionic [[nicotinic acetylcholine receptor]] present in [[Sympathetic nervous system|sympathetic]], [[Parasympathetic nervous system|parasympathetic]], and [[Enteric nervous system|enteric ganglia]]. Typical symptoms include [[Intestinal pseudo-obstruction|gastrointestinal dysmotility]], [[orthostatic hypotension]], and [[Adie syndrome|tonic pupils]].<ref name="Winston Vernino 2009 pp. 85–93">{{cite book | last=Winston | first=Nicole | last2=Vernino | first2=Steven | title=Frontiers of Neurology and Neuroscience | chapter=Autoimmune Autonomic Ganglionopathy | publisher=KARGER | publication-place=Basel | year=2009 | issn=1660-4431 | doi=10.1159/000212370 | page=85–93}}</ref> Many cases have a sudden onset, but others worsen over time, resembling degenerative forms of [[Dysautonomia|autonomic dysfunction]]. For milder cases, supportive treatment is used to manage symptoms.<ref name="Wang Low Jordan Freeman 2007 pp. 1917–1921">{{cite journal | last=Wang | first=Z. | last2=Low | first2=P. A. | last3=Jordan | first3=J. | last4=Freeman | first4=R. | last5=Gibbons | first5=C. H. | last6=Schroeder | first6=C. | last7=Sandroni | first7=P. | last8=Vernino | first8=S. | title=Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current | journal=Neurology | publisher=Ovid Technologies (Wolters Kluwer Health) | volume=68 | issue=22 | date=May 29, 2007 | issn=0028-3878 | doi=10.1212/01.wnl.0000263185.30294.61 | pages=1917–1921}}</ref> [[Plasmapheresis|Plasma exchange]], intravenous [[Immunoglobulin therapy|immunoglobulin]], [[ |
'''Autoimmune autonomic ganglionopathy''' is a type of immune-mediated autonomic failure that is associated with antibodies against the ganglionic [[nicotinic acetylcholine receptor]] present in [[Sympathetic nervous system|sympathetic]], [[Parasympathetic nervous system|parasympathetic]], and [[Enteric nervous system|enteric ganglia]]. Typical symptoms include [[Intestinal pseudo-obstruction|gastrointestinal dysmotility]], [[orthostatic hypotension]], and [[Adie syndrome|tonic pupils]].<ref name="Winston Vernino 2009 pp. 85–93">{{cite book | last=Winston | first=Nicole | last2=Vernino | first2=Steven | title=Frontiers of Neurology and Neuroscience | chapter=Autoimmune Autonomic Ganglionopathy | publisher=KARGER | publication-place=Basel | year=2009 | issn=1660-4431 | doi=10.1159/000212370 | page=85–93}}</ref> Many cases have a sudden onset, but others worsen over time, resembling degenerative forms of [[Dysautonomia|autonomic dysfunction]]. For milder cases, supportive treatment is used to manage symptoms.<ref name="Wang Low Jordan Freeman 2007 pp. 1917–1921">{{cite journal | last=Wang | first=Z. | last2=Low | first2=P. A. | last3=Jordan | first3=J. | last4=Freeman | first4=R. | last5=Gibbons | first5=C. H. | last6=Schroeder | first6=C. | last7=Sandroni | first7=P. | last8=Vernino | first8=S. | title=Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current | journal=Neurology | publisher=Ovid Technologies (Wolters Kluwer Health) | volume=68 | issue=22 | date=May 29, 2007 | issn=0028-3878 | doi=10.1212/01.wnl.0000263185.30294.61 | pages=1917–1921}}</ref> [[Plasmapheresis|Plasma exchange]], intravenous [[Immunoglobulin therapy|immunoglobulin]], [[corticosteroid]]s, or [[immunosuppression]] have been used successfully to treat more severe cases.<ref name="Winston Vernino 2009 pp. 85–93"/> |
||
== Signs and symptoms == |
== Signs and symptoms == |
||
| Line 52: | Line 52: | ||
[[Constipation]] or [[diarrhea]], [[Vomiting|emesis]], [[Anorexia (symptom)|anorexia]], early [[satiety]], and [[abdominal pain]] are common symptoms of gastrointestinal dysmotility, which affects 70% of patients.<ref name="Winston Vernino 2009 pp. 85–93"/> |
[[Constipation]] or [[diarrhea]], [[Vomiting|emesis]], [[Anorexia (symptom)|anorexia]], early [[satiety]], and [[abdominal pain]] are common symptoms of gastrointestinal dysmotility, which affects 70% of patients.<ref name="Winston Vernino 2009 pp. 85–93"/> |
||
Although about a quarter of patients report neuropathic symptoms such as tingling in the distal extremities, sensory examination and nerve conduction studies are normal.<ref name="Winston Vernino 2009 pp. 85–93"/> |
Although about a quarter of patients report neuropathic symptoms such as tingling in the distal extremities, sensory examination and nerve conduction studies are normal.<ref name="Winston Vernino 2009 pp. 85–93"/> |
||
=== Complications === |
=== Complications === |
||
In severe cases, [[intestinal pseudo-obstruction]] can result in death. Patients frequently lose weight as a result of their decreased ability to maintain nutrition.<ref name="Winston Vernino 2009 pp. 85–93"/> |
In severe cases, [[intestinal pseudo-obstruction]] can result in death. Patients frequently lose weight as a result of their decreased ability to maintain nutrition.<ref name="Winston Vernino 2009 pp. 85–93"/> |
||
== Causes == |
== Causes == |
||
| Line 63: | Line 63: | ||
Antibodies against the neuronal [[nicotinic acetylcholine receptor]] found in [[Autonomic ganglion|autonomic ganglia]] are present in approximately 50% of AAG patients. The ganglionic [[Acetylcholine receptor|AChR]], which is homologous but genetically and immunologically distinct from the AChR at the [[neuromuscular junction]], mediates fast [[Neurotransmission|synaptic transmission]] in all [[Autonomic ganglion|autonomic ganglia]].<ref name="Wang Low Jordan Freeman 2007 pp. 1917–1921">{{cite journal | last=Wang | first=Z. | last2=Low | first2=P. A. | last3=Jordan | first3=J. | last4=Freeman | first4=R. | last5=Gibbons | first5=C. H. | last6=Schroeder | first6=C. | last7=Sandroni | first7=P. | last8=Vernino | first8=S. | title=Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current | journal=Neurology | publisher=Ovid Technologies (Wolters Kluwer Health) | volume=68 | issue=22 | date=May 29, 2007 | issn=0028-3878 | doi=10.1212/01.wnl.0000263185.30294.61 | pages=1917–1921}}</ref> |
Antibodies against the neuronal [[nicotinic acetylcholine receptor]] found in [[Autonomic ganglion|autonomic ganglia]] are present in approximately 50% of AAG patients. The ganglionic [[Acetylcholine receptor|AChR]], which is homologous but genetically and immunologically distinct from the AChR at the [[neuromuscular junction]], mediates fast [[Neurotransmission|synaptic transmission]] in all [[Autonomic ganglion|autonomic ganglia]].<ref name="Wang Low Jordan Freeman 2007 pp. 1917–1921">{{cite journal | last=Wang | first=Z. | last2=Low | first2=P. A. | last3=Jordan | first3=J. | last4=Freeman | first4=R. | last5=Gibbons | first5=C. H. | last6=Schroeder | first6=C. | last7=Sandroni | first7=P. | last8=Vernino | first8=S. | title=Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current | journal=Neurology | publisher=Ovid Technologies (Wolters Kluwer Health) | volume=68 | issue=22 | date=May 29, 2007 | issn=0028-3878 | doi=10.1212/01.wnl.0000263185.30294.61 | pages=1917–1921}}</ref> |
||
Several lines of evidence have shown that 3 gAChR antibodies are pathogenic in AAG. In vitro, [[Immunoglobulin G|IgG]] from AAG patients reduces gAChR current in cultured neuroblastoma cells. Mice with genetically engineered [[ |
Several lines of evidence have shown that 3 gAChR antibodies are pathogenic in AAG. In vitro, [[Immunoglobulin G|IgG]] from AAG patients reduces gAChR current in cultured neuroblastoma cells. Mice with genetically engineered [[null mutation]]s in the 3 [[Protein subunit|subunit]] have reduced autonomic ganglionic transmission, resulting in [[urinary retention]], dilated and nonreactive pupils, and higher mortality rates. Active immunization of rabbits against the 3 subunit results in gastrointestinal hypomotility, urinary retention, and a reduced number of gACh receptors on postsynaptic ganglionic neurons, as well as impaired synaptic transmission.<ref name="Golden Vernino 2019 pp. 277–288">{{cite journal | last=Golden | first=Elisabeth P. | last2=Vernino | first2=Steven | title=Autoimmune autonomic neuropathies and ganglionopathies: epidemiology, pathophysiology, and therapeutic advances | journal=Clinical Autonomic Research | publisher=Springer Science and Business Media LLC | volume=29 | issue=3 | date=May 15, 2019 | issn=0959-9851 | doi=10.1007/s10286-019-00611-1 | pages=277–288}}</ref> |
||
== Diagnosis == |
== Diagnosis == |
||
After ruling out other etiologies, the diagnosis of AAG is made based on clinical indicators. In as many as 50% of individuals with classic AAG symptoms, serum ganglionic neuronal nicotinic [[AChR]] antibodies are detected. A negative test does not rule out the diagnosis; however, a positive blood AChR antibody is specific for AAG. When a patient has subacute, severe symptoms or has cancer risk factors, screening [[CT scan|computed tomography]] of the chest is indicated. Some individuals with [[Paraneoplastic syndrome|paraneoplastic autonomic neuropathy]] have ganglionic AChR antibodies, therefore occult [[Small-cell carcinoma|small cell carcinoma]] or [[thymoma]] should be explored.<ref name="Winston Vernino 2009 pp. 85–93"/> |
|||
Traditional autonomic testing is used to aid in the diagnosis of AAG. These tests can include a [[tilt table test]] (TTT), thermoregulatory sweat test (TST), quantitative sudomotor autonomic reflex testing (QSART) and various blood panels. Additionally, a blood test showing high levels of the antibody ganglionic nicotenic acetylcholine receptor (gAChr) occur in about 50% of patients with AAG (seropositive AAG). The seronegative patients (those without detectable gAChR levels) are theorized to have one or more different antibodies responsible for the autonomic dysfunction. However, both seropositive and seronegative patients have been seen to respond to the same treatments. A paraneoplastic panel may also be ordered to rule out paraneoplastic syndrome.<ref>{{cite book| chapter-url=https://mayoclinic.pure.elsevier.com/en/publications/autoimmune-autonomic-ganglionopathy | chapter=Autoimmune Autonomic Ganglionopathy| doi=10.1016/B978-0-12-386525-0.00100-1| title=Primer on the Autonomic Nervous System| pages=489–492| year=2012| last1=Vernino| first1=Steven| last2=Low| first2=Phillip A.| isbn=9780123865250| citeseerx=10.1.1.657.2841}}</ref> |
|||
=== Differential diagnosis === |
|||
The most common differential diagnosis is [[Paraneoplastic syndrome|paraneoplastic autonomic neuropathy]], which can mimic AAG symptoms. [[Orthostatic hypotension]] and significant gastrointestinal symptoms are also hallmarks of paraneoplastic [[dysautonomia]]. Paraneoplastic dysautonomia has been linked to [[thymoma]], [[Small-cell carcinoma|small-cell lung carcinoma]], and, less frequently, [[breast cancer]] or [[lymphoma]]. At the time of the development of autonomic symptoms, the underlying malignancy is usually unknown. Antibody testing, such as anti-Hu or [[Collapsin response mediator protein family|collapsing response mediator protein]], can aid in the identification of paraneoplastic patients.<ref name="Winston Vernino 2009 pp. 85–93"/> |
|||
One should rule out [[Guillain–Barré syndrome|Guillain-Barré syndrome]] if the patient exhibits acute or subacute autonomic instability accompanied by [[weakness]], as this condition frequently results in [[ileus]], [[constipation]], and blood pressure swings. If, on the other hand, the patient has autonomic overactivity with [[muscle stiffness]] and spontaneous [[Fasciculation|muscle twitching]], an autoimmune [[neuromyotonia]] or [[Morvan's syndrome|Morvan syndrome]] diagnosis may be considered.<ref name="Winston Vernino 2009 pp. 85–93"/> |
|||
Chronic and progressive onset of autonomic symptoms may indicate [[diabetes]], [[amyloidosis]], or [[Sjögren syndrome|Sjogren's syndrome]]. AAG can be difficult to distinguish from degenerative autonomic disorders such as [[pure autonomic failure]] or [[multiple system atrophy]] when autonomic symptoms appear gradually. When the time course is unknown, the presence of prominent gastrointestinal dysmotility and impaired pupillary light reflexes should point to AAG.<ref name="Winston Vernino 2009 pp. 85–93"/> |
|||
== Treatment == |
== Treatment == |
||
Where an underlying [[neoplasm]] is the cause, treatment of this condition is indicated to reduce progression of symptoms. For cases without a known cause, treatment involves suppression of the immune system with [[corticosteroid]] treatment, [[intravenous immunoglobulin]], immunosuppressive agents like [[rituximab]], [[Mycophenolic acid|mycophenolate mofetil]] (Cellcept), or [[azathioprine]] (Imuran) or [[plasmapheresis]].<ref name="Therapy">{{cite journal|doi=10.1001/archneurol.2007.60|pmid=18268189|author=Christopher H. Gibbons, MD, MMSc|author2=Steven A. Vernino, MD|author3=Roy Freeman, MD |name-list-style=amp |journal=Arch. Neurol.|volume=65|issue=2|pages=213–217|year=2007|title=Combined Immunomodulatory Therapy in Autoimmune Autonomic Ganglionopathy|doi-access=}}</ref> |
Where an underlying [[neoplasm]] is the cause, treatment of this condition is indicated to reduce progression of symptoms. For cases without a known cause, treatment involves suppression of the immune system with [[corticosteroid]] treatment, [[intravenous immunoglobulin]], immunosuppressive agents like [[rituximab]], [[Mycophenolic acid|mycophenolate mofetil]] (Cellcept), or [[azathioprine]] (Imuran) or [[plasmapheresis]].<ref name="Therapy">{{cite journal|doi=10.1001/archneurol.2007.60|pmid=18268189|author=Christopher H. Gibbons, MD, MMSc|author2=Steven A. Vernino, MD|author3=Roy Freeman, MD |name-list-style=amp |journal=Arch. Neurol.|volume=65|issue=2|pages=213–217|year=2007|title=Combined Immunomodulatory Therapy in Autoimmune Autonomic Ganglionopathy|doi-access=}}</ref> |
||
== Outlook == |
|||
The ganglionic AChR antibody level in AAG patients corresponds with the degree of severity of autonomic signs and symptoms. Patients with high levels of ganglionic AChR antibodies frequently appear with subacute onset and significant cholinergic dysautonomia.<ref name="Winston Vernino 2009 pp. 85–93"/> |
|||
== See also == |
== See also == |
||
| Line 79: | Line 89: | ||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
== Further reading == |
|||
* {{cite journal | last=Nakane | first=Shunya | last2=Mukaino | first2=Akihiro | last3=Higuchi | first3=Osamu | last4=Watari | first4=Mari | last5=Maeda | first5=Yasuhiro | last6=Yamakawa | first6=Makoto | last7=Nakahara | first7=Keiichi | last8=Takamatsu | first8=Koutaro | last9=Matsuo | first9=Hidenori | last10=Ando | first10=Yukio | title=Autoimmune autonomic ganglionopathy: an update on diagnosis and treatment | journal=Expert Review of Neurotherapeutics | publisher=Informa UK Limited | volume=18 | issue=12 | year=2018 | issn=1473-7175 | doi=10.1080/14737175.2018.1540304 | pages=953–965|ref=none}} |
|||
* {{cite journal | last=Nakane | first=Shunya | last2=Mukaino | first2=Akihiro | last3=Higuchi | first3=Osamu | last4=Yasuhiro | first4=Maeda | last5=Takamatsu | first5=Koutaro | last6=Yamakawa | first6=Makoto | last7=Watari | first7=Mari | last8=Tawara | first8=Nozomu | last9=Nakahara | first9=Kei-ichi | last10=Kawakami | first10=Atsushi | last11=Matsuo | first11=Hidenori | last12=Ando | first12=Yukio | title=A comprehensive analysis of the clinical characteristics and laboratory features in 179 patients with autoimmune autonomic ganglionopathy | journal=Journal of Autoimmunity | publisher=Elsevier BV | volume=108 | year=2020 | issn=0896-8411 | doi=10.1016/j.jaut.2020.102403 | page=102403|ref=none}} |
|||
* {{cite journal | last=Nakane | first=Shunya | last2=Higuchi | first2=Osamu | last3=Koga | first3=Michiaki | last4=Kanda | first4=Takashi | last5=Murata | first5=Kenya | last6=Suzuki | first6=Takashi | last7=Kurono | first7=Hiroko | last8=Kunimoto | first8=Masanari | last9=Kaida | first9=Ken-ichi | last10=Mukaino | first10=Akihiro | last11=Sakai | first11=Waka | last12=Maeda | first12=Yasuhiro | last13=Matsuo | first13=Hidenori | title=Clinical Features of Autoimmune Autonomic Ganglionopathy and the Detection of Subunit-Specific Autoantibodies to the Ganglionic Acetylcholine Receptor in Japanese Patients | journal=PLOS ONE | publisher=Public Library of Science (PLoS) | volume=10 | issue=3 | date=2015-03-19 | issn=1932-6203 | doi=10.1371/journal.pone.0118312 | page=e0118312|ref=none}} |
|||
* {{cite journal | last=Parize | first=P. | last2=Gaultier | first2=J.-B. | last3=Badet | first3=F. | last4=André-Obadia | first4=N. | last5=Dupond | first5=J.-L. | last6=Rousset | first6=H. | last7=Durieu | first7=I. | title=Autoimmune autonomic ganglionopathy: A case series of six patients and literature review | publisher=Elsevier BV | volume=31 | issue=7 | year=2010 | issn=0248-8663 | doi=10.1016/j.revmed.2010.01.008 | pages=476–480 | language=fr|ref=none}} |
|||
* {{cite book | last=Nakane | first=Shunya | last2=Higuchi | first2=Osamu | last3=Matsuo | first3=Hidenori | title=Neuroimmunological Diseases | chapter=Autoimmune Autonomic Ganglionopathy | publisher=Springer Japan | publication-place=Tokyo | year=2016 | isbn=978-4-431-55593-3 | doi=10.1007/978-4-431-55594-0_17|ref=none}} |
|||
== External links == |
== External links == |
||
Revision as of 02:57, 4 November 2023
| Autoimmune autonomic ganglionopathy | |
|---|---|
| Other names | Autoimmune autonomic neuropathy, Acute pandysautonomia |
 | |
| Functions of the autonomic nervous system. | |
| Specialty | Neurology |
| Symptoms | Gastrointestinal dysmotility, orthostatic hypotension, and tonic pupils.[1] |
| Complications | Weight loss. |
| Diagnostic method | Clinical criteria and serum ganglionic neuronal nicotinic AChR antibodies. |
| Differential diagnosis | Paraneoplastic syndrome, Guillain-Barré syndrome, diabetes, amyloidosis, Sjogren's syndrome, and Morvan syndrome. |
Autoimmune autonomic ganglionopathy is a type of immune-mediated autonomic failure that is associated with antibodies against the ganglionic nicotinic acetylcholine receptor present in sympathetic, parasympathetic, and enteric ganglia. Typical symptoms include gastrointestinal dysmotility, orthostatic hypotension, and tonic pupils.[1] Many cases have a sudden onset, but others worsen over time, resembling degenerative forms of autonomic dysfunction. For milder cases, supportive treatment is used to manage symptoms.[2] Plasma exchange, intravenous immunoglobulin, corticosteroids, or immunosuppression have been used successfully to treat more severe cases.[1]
Signs and symptoms
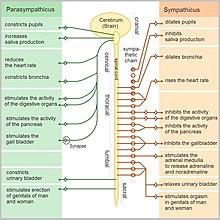
Individuals symptoms vary in severity and type. Severe, subacute gastrointestinal dysmotility and orthostatic hypotension are the most common symptoms in two-thirds of patients. Symptoms can be severe in some cases and gradually worsen in others.[1]
Sympathetic failure manifests itself as orthostatic hypotension and anhidrosis. Orthostatic symptoms, which include lightheadedness, dizziness, or syncope upon standing and loss of postural reflex tachycardia, occur in 78% of patients.[1]
Dry eyes and mouth are symptoms of parasympathetic failure caused by secretomotor dysfunction. Additional symptoms of the parasympathetic nervous system include constipation, light sensitivity, impotence, and retention of urine. A minimal change in heart rate during deep breathing, or the Valsalva maneuver, and resting tachycardia may occur when the heart's cardiovagal control fails.[1]
Constipation or diarrhea, emesis, anorexia, early satiety, and abdominal pain are common symptoms of gastrointestinal dysmotility, which affects 70% of patients.[1]
Although about a quarter of patients report neuropathic symptoms such as tingling in the distal extremities, sensory examination and nerve conduction studies are normal.[1]
Complications
In severe cases, intestinal pseudo-obstruction can result in death. Patients frequently lose weight as a result of their decreased ability to maintain nutrition.[1]
Causes
The cause is generally either paraneoplastic syndrome or idiopathic. In idiopathic AAG, the body's own immune system targets a receptor in the autonomic ganglia, which is part of a peripheral nerve fiber. If the AAG is paraneoplastic, they have a form of cancer, and their immune system has produced paraneoplastic antibodies in response to the cancer.[3]
Pathophysiology
Antibodies against the neuronal nicotinic acetylcholine receptor found in autonomic ganglia are present in approximately 50% of AAG patients. The ganglionic AChR, which is homologous but genetically and immunologically distinct from the AChR at the neuromuscular junction, mediates fast synaptic transmission in all autonomic ganglia.[2]
Several lines of evidence have shown that 3 gAChR antibodies are pathogenic in AAG. In vitro, IgG from AAG patients reduces gAChR current in cultured neuroblastoma cells. Mice with genetically engineered null mutations in the 3 subunit have reduced autonomic ganglionic transmission, resulting in urinary retention, dilated and nonreactive pupils, and higher mortality rates. Active immunization of rabbits against the 3 subunit results in gastrointestinal hypomotility, urinary retention, and a reduced number of gACh receptors on postsynaptic ganglionic neurons, as well as impaired synaptic transmission.[4]
Diagnosis
After ruling out other etiologies, the diagnosis of AAG is made based on clinical indicators. In as many as 50% of individuals with classic AAG symptoms, serum ganglionic neuronal nicotinic AChR antibodies are detected. A negative test does not rule out the diagnosis; however, a positive blood AChR antibody is specific for AAG. When a patient has subacute, severe symptoms or has cancer risk factors, screening computed tomography of the chest is indicated. Some individuals with paraneoplastic autonomic neuropathy have ganglionic AChR antibodies, therefore occult small cell carcinoma or thymoma should be explored.[1]
Differential diagnosis
The most common differential diagnosis is paraneoplastic autonomic neuropathy, which can mimic AAG symptoms. Orthostatic hypotension and significant gastrointestinal symptoms are also hallmarks of paraneoplastic dysautonomia. Paraneoplastic dysautonomia has been linked to thymoma, small-cell lung carcinoma, and, less frequently, breast cancer or lymphoma. At the time of the development of autonomic symptoms, the underlying malignancy is usually unknown. Antibody testing, such as anti-Hu or collapsing response mediator protein, can aid in the identification of paraneoplastic patients.[1]
One should rule out Guillain-Barré syndrome if the patient exhibits acute or subacute autonomic instability accompanied by weakness, as this condition frequently results in ileus, constipation, and blood pressure swings. If, on the other hand, the patient has autonomic overactivity with muscle stiffness and spontaneous muscle twitching, an autoimmune neuromyotonia or Morvan syndrome diagnosis may be considered.[1]
Chronic and progressive onset of autonomic symptoms may indicate diabetes, amyloidosis, or Sjogren's syndrome. AAG can be difficult to distinguish from degenerative autonomic disorders such as pure autonomic failure or multiple system atrophy when autonomic symptoms appear gradually. When the time course is unknown, the presence of prominent gastrointestinal dysmotility and impaired pupillary light reflexes should point to AAG.[1]
Treatment
Where an underlying neoplasm is the cause, treatment of this condition is indicated to reduce progression of symptoms. For cases without a known cause, treatment involves suppression of the immune system with corticosteroid treatment, intravenous immunoglobulin, immunosuppressive agents like rituximab, mycophenolate mofetil (Cellcept), or azathioprine (Imuran) or plasmapheresis.[5]
Outlook
The ganglionic AChR antibody level in AAG patients corresponds with the degree of severity of autonomic signs and symptoms. Patients with high levels of ganglionic AChR antibodies frequently appear with subacute onset and significant cholinergic dysautonomia.[1]
See also
References
- ^ a b c d e f g h i j k l m n Winston, Nicole; Vernino, Steven (2009). "Autoimmune Autonomic Ganglionopathy". Frontiers of Neurology and Neuroscience. Basel: KARGER. p. 85–93. doi:10.1159/000212370. ISSN 1660-4431.
- ^ a b Wang, Z.; Low, P. A.; Jordan, J.; Freeman, R.; Gibbons, C. H.; Schroeder, C.; Sandroni, P.; Vernino, S. (May 29, 2007). "Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current". Neurology. 68 (22). Ovid Technologies (Wolters Kluwer Health): 1917–1921. doi:10.1212/01.wnl.0000263185.30294.61. ISSN 0028-3878.
- ^ Paola Sandroni & Phillip A. Low (2009). "Other Autonomic Neuropathies Associated with Ganglionic Antibody". Autonomic Neuroscience. 146 (1–2): 13–17. doi:10.1016/j.autneu.2008.10.022. PMC 2671239. PMID 19058765.
- ^ Golden, Elisabeth P.; Vernino, Steven (May 15, 2019). "Autoimmune autonomic neuropathies and ganglionopathies: epidemiology, pathophysiology, and therapeutic advances". Clinical Autonomic Research. 29 (3). Springer Science and Business Media LLC: 277–288. doi:10.1007/s10286-019-00611-1. ISSN 0959-9851.
- ^ Christopher H. Gibbons, MD, MMSc; Steven A. Vernino, MD & Roy Freeman, MD (2007). "Combined Immunomodulatory Therapy in Autoimmune Autonomic Ganglionopathy". Arch. Neurol. 65 (2): 213–217. doi:10.1001/archneurol.2007.60. PMID 18268189.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Nakane, Shunya; Mukaino, Akihiro; Higuchi, Osamu; Watari, Mari; Maeda, Yasuhiro; Yamakawa, Makoto; Nakahara, Keiichi; Takamatsu, Koutaro; Matsuo, Hidenori; Ando, Yukio (2018). "Autoimmune autonomic ganglionopathy: an update on diagnosis and treatment". Expert Review of Neurotherapeutics. 18 (12). Informa UK Limited: 953–965. doi:10.1080/14737175.2018.1540304. ISSN 1473-7175.
- Nakane, Shunya; Mukaino, Akihiro; Higuchi, Osamu; Yasuhiro, Maeda; Takamatsu, Koutaro; Yamakawa, Makoto; Watari, Mari; Tawara, Nozomu; Nakahara, Kei-ichi; Kawakami, Atsushi; Matsuo, Hidenori; Ando, Yukio (2020). "A comprehensive analysis of the clinical characteristics and laboratory features in 179 patients with autoimmune autonomic ganglionopathy". Journal of Autoimmunity. 108. Elsevier BV: 102403. doi:10.1016/j.jaut.2020.102403. ISSN 0896-8411.
- Nakane, Shunya; Higuchi, Osamu; Koga, Michiaki; Kanda, Takashi; Murata, Kenya; Suzuki, Takashi; Kurono, Hiroko; Kunimoto, Masanari; Kaida, Ken-ichi; Mukaino, Akihiro; Sakai, Waka; Maeda, Yasuhiro; Matsuo, Hidenori (2015-03-19). "Clinical Features of Autoimmune Autonomic Ganglionopathy and the Detection of Subunit-Specific Autoantibodies to the Ganglionic Acetylcholine Receptor in Japanese Patients". PLOS ONE. 10 (3). Public Library of Science (PLoS): e0118312. doi:10.1371/journal.pone.0118312. ISSN 1932-6203.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Parize, P.; Gaultier, J.-B.; Badet, F.; André-Obadia, N.; Dupond, J.-L.; Rousset, H.; Durieu, I. (2010). "Autoimmune autonomic ganglionopathy: A case series of six patients and literature review" (in French). 31 (7). Elsevier BV: 476–480. doi:10.1016/j.revmed.2010.01.008. ISSN 0248-8663.
{{cite journal}}: Cite journal requires|journal=(help) - Nakane, Shunya; Higuchi, Osamu; Matsuo, Hidenori (2016). "Autoimmune Autonomic Ganglionopathy". Neuroimmunological Diseases. Tokyo: Springer Japan. doi:10.1007/978-4-431-55594-0_17. ISBN 978-4-431-55593-3.
