Photoinhibition: Difference between revisions
move quantum yield to a sub section, other minor adjustments |
dois and isbn |
||
| Line 1: | Line 1: | ||
[[File:Photoinhibition.svg|thumb|right|280px|Photoinhibition of [[Photosystem II]] (PSII) leads to loss of PSII electron transfer activity. PSII is continuously repaired via degradation and synthesis of the D1 protein. [[Lincomycin]] can be used to block protein synthesis]] |
[[File:Photoinhibition.svg|thumb|right|280px|Photoinhibition of [[Photosystem II]] (PSII) leads to loss of PSII electron transfer activity. PSII is continuously repaired via degradation and synthesis of the D1 protein. [[Lincomycin]] can be used to block protein synthesis]] |
||
'''Photoinhibition''' is light-induced reduction in the [[photosynthesis|photosynthetic]] capacity of a [[plant]], [[algae|alga]] or a [[cyanobacteria|cyanobacterium]]. [[Photosystem II]] (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the [[photosynthetic reaction center]] of PSII. Photoinhibition is also been used in a wider sense, as [[Photoinhibition#Dynamic_photoinhibition|dynamic photoinhibition]], to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light. Several recent reviews on photoinhibition are available.<ref name="Vass and Cser 2009">{{cite journal|author = Vass I & Cser K|title= Janus-faced charge recombinations in photosystem II photoinhibition|journal=Trends in Plant Science|volume=14|issue=4|year=2009|pages=200–205}}</ref><ref name="Tyystjarvi 2008">{{cite journal|author = Tyystjärvi E|title= Photoinhibition of Photosystem II and photodamage of the oxygen-evolving manganese cluster|journal=Coordination Chemistry Reviews|volume=252|issue=3–4|year=2008|pages=361–376|doi=10.1016/j.ccr.2007.08.021}}</ref><ref name="Krieger-Liszkay et al 2008">{{cite journal|author = Krieger-Liszkay A, Fufezan C & Trebst A|title= Singlet oxygen production in photosystem II and related protection mechanism|journal=Photosynthesis Research|volume=98|issue=1-3|year=2008|pages=551–564}}</ref><ref name="Mohanty et al 2008">{{cite journal|author =Mohanty P, Allakhverdiev SI & Murata N|title= Application of low temperatures during photoinhibition allows characterization of individual steps in photodamage and the repair of photosystem II|journal=Photosynthesis Research|volume=94|issue=2-3|year=2007|pages=217–224}}</ref><ref name="Nishiyama et al 2006">{{cite journal|author = Nishiyama Y, Allakhverdiev SI & Murata N|title= A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II |journal=Biophysica et Biochimica Acta - Bioenergetics|volume=1757|issue=7|year=2006|pages=742–749}}</ref><ref name="Telfer 2005">{{cite journal|author = Telfer A|title= Too much light? How beta-carotene protects the photosystem II reaction centre|journal=Photochemical & Photobiological Sciences|volume=4|issue=12|year=2005|pages=950–956}}</ref><ref name="Adir et al 2003">{{cite journal|author =Adir N, Zer H, Shochat S & Ohad I|title=Photoinhibition - a historical perspective|journal=Photosynthesis Research|volume=76|issue=1-3|year=2003|pages=343–370}}</ref><ref name="Niyogi 1999">{{cite journal|author =Niyogi KK|title=Photoprotection revisited: Genetic and molecular approaches|journal=Annual Review of Plant Physiology and Plant Molecular Biology|volume=50|year=1999|pages=333–359}}</ref> |
'''Photoinhibition''' is light-induced reduction in the [[photosynthesis|photosynthetic]] capacity of a [[plant]], [[algae|alga]] or a [[cyanobacteria|cyanobacterium]]. [[Photosystem II]] (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the [[photosynthetic reaction center]] of PSII. Photoinhibition is also been used in a wider sense, as [[Photoinhibition#Dynamic_photoinhibition|dynamic photoinhibition]], to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light. Several recent reviews on photoinhibition are available.<ref name="Vass and Cser 2009"> |
||
{{cite journal|author = Vass I & Cser K|title= Janus-faced charge recombinations in photosystem II photoinhibition|journal=Trends in Plant Science|volume=14|issue=4|year=2009|pages=200–205 |doi = 10.1016/j.tplants.2009.01.009}}</ref><ref name="Tyystjarvi 2008"> |
|||
{{cite journal|author = Tyystjärvi E|title= Photoinhibition of Photosystem II and photodamage of the oxygen-evolving manganese cluster|journal=Coordination Chemistry Reviews|volume=252|issue=3–4|year=2008|pages=361–376|doi=10.1016/j.ccr.2007.08.021}}</ref><ref name="Krieger-Liszkay et al 2008"> |
|||
{{cite journal|author = Krieger-Liszkay A, Fufezan C & Trebst A|title= Singlet oxygen production in photosystem II and related protection mechanism|journal=Photosynthesis Research|volume=98|issue=1-3|year=2008|pages=551–564|doi = 10.1007/s11120-008-9349-3}}</ref><ref name="Mohanty et al 2008"> |
|||
{{cite journal|author =Mohanty P, Allakhverdiev SI & Murata N|title= Application of low temperatures during photoinhibition allows characterization of individual steps in photodamage and the repair of photosystem II|journal=Photosynthesis Research|volume=94|issue=2-3|year=2007|pages=217–224|doi = 10.1007/s11120-007-9184-y}}</ref><ref name="Nishiyama et al 2006"> |
|||
{{cite journal|author = Nishiyama Y, Allakhverdiev SI & Murata N|title= A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II |journal=Biophysica et Biochimica Acta - Bioenergetics|volume=1757|issue=7|year=2006|pages=742–749|doi=10.1016/j.bbabio.2006.05.013}}</ref><ref name="Telfer 2005"> |
|||
{{cite journal|author = Telfer A|title= Too much light? How beta-carotene protects the photosystem II reaction centre|journal=Photochemical & Photobiological Sciences|volume=4|issue=12|year=2005|pages=950–956|doi=10.1039/b507888c}}</ref><ref name="Adir et al 2003"> |
|||
{{cite journal|author =Adir N, Zer H, Shochat S & Ohad I|title=Photoinhibition - a historical perspective|journal=Photosynthesis Research|volume=76|issue=1-3|year=2003|pages=343–370|doi=10.1007/1-4020-3324-9}}</ref><ref name="Niyogi 1999"> |
|||
{{cite journal|author =Niyogi KK|title=Photoprotection revisited: Genetic and molecular approaches|journal=Annual Review of Plant Physiology and Plant Molecular Biology|volume=50|year=1999|pages=333–359|doi=10.1146/annurev.arplant.50.1.333}}</ref> |
|||
==History== |
==History== |
||
The first measurements of photoinhibition were published in 1956 by Bessel Kok.<ref>{{cite journal|author = Kok B|title= On the inhibition of photosynthesis by intense light |journal=Biochimica et Biophysica Acta|volume=21|issue=2|year=1956|pages=234–244|doi=10.1016/0006-3002(56)90003-8}}</ref> Even in the very first studies it became obvious that plants have a repair mechanism that continuously repairs photoinhibitory damage. In 1966, Jones and Kok measured the action spectrum of photoinhibition and found that ultraviolet light is highly photoinhibitory.<ref name="Jones 1966">{{cite journal|author = Jones LW & Kok B|title= Photoinhibition of chloroplast reactions. I. Kinetics and action spectra |journal=Plant Physiology|volume=41|issue=6|year=1966|pages=1037–1043}}</ref> The visible-light part of the action spectrum was found to have a peak in the red-light region, suggesting that chlorophylls act as photoreceptors of photoinhibition. In the 1980s, photoinhibition became a popular topic in photosynthesis research, and the concept of a damaging reaction counteracted by a repair process was re-invented. Research was stimulated by a paper by Kyle, Ohad and Arntzen in 1984, showing that photoinhibition is accompanied by selective loss of a 32-kDa protein, later identified as the PSII reaction center protein D1.<ref name="Kyle 1984">{{cite journal|author = Kyle DJ, Ohad I, Arntzen CJ|title= Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes|journal=Proceedings of the National Academy of Sciences of the USA|volume=81|issue=13|year=1984|pages=4070–4074}}</ref> The photosensitivity of PSII from which the oxygen evolving complex had been inactivated with chemical treatment was studied in the 1980s and early 1990s.<ref name = "Callahan et al 1986">{{cite journal|author = Callahan FE, Becker DW & Cheniae GM|title= Studies on the photoactivation of the water-oxidizing enzyme. II. Characterization of weak light photoinhibition of PSII and its light-induced recovery|journal=Plant Physiology|volume=82| year=1986|pages=261–269}}</ref><ref>{{cite journal|author = Jegerschöld C, Virgin I & Styring S|title= Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction|journal=Biochemistry|volume=29|year=1990|pages=6179–6186}}</ref> A paper by Imre Vass and coworkers in 1992 described the acceptor-side mechanism of photoinhibition.<ref name = "Vass et al 1992">{{cite journal|author = Vass I, Styring S, Hundal T, Koivuniemi M, Aro E-M & Andersson B|title=Reversible and irreversible intermediates during photoinhibition of photosystem II: Stable reduced Q<sub>A</sub> species promote chlorophyll triplet formation|journal=Proceedings of the National Academy of Sciences of the USA|volume=89| year=1992|pages=1408–1412}}</ref> Measurements of production of [[singlet oxygen]] by photoinhibited PSII provided further evidence for an acceptor-side-type mechanism.<ref name="Hideg et al 1998">{{cite journal|author = Hideg É, Kálai T, Hideg K & Vass I|title= Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves|journal=Biochemistry|volume=37|issue=33|year=1998|pages=11405–11411|doi=10.1021/bi972890+}}</ref> The concept of a repair cycle that continuously repairs photoinhibitory damage, evolved and was reviewed by Aro et al. in 1993.<ref name="Aro et al 1993">{{cite journal|author = Aro E-M, Virgin I & Andersson B|title= Photoinhibition of Photosystem II – inactivation, protein damage and turnover|journal=Biophysica et Biochimica Acta |volume=1143|issue=2|year=1993|pages=113–134}}</ref> Many details of the repair cycle, including the finding that the FtsH [[protease]] plays an important role in the degradation of the D1 protein, have been discovered since.<ref name="Bailey et al 2002">{{cite journal|author = Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C & Mann NH|title=A critical role for the Var2 FtsH homologue of ''Arabidopsis thaliana'' in the Photosystem II repair cycle in vivo|journal=Journal of Biological Chemistry|volume=277|issue=3|year=2002|pages=2006–2011|doi=10.1074/jbc.M105878200}}</ref> In 1996, a paper by Tyystjärvi and Aro showed that that the rate constant of photoinhibition is directly proportional to light intensity, a result that opposed the former assumption that photoinhibition is caused by the fraction of light energy that exceeds the maximum capability of photosynthesis.<ref name="Tyystjarvi and Aro 1996">{{cite journal|author = Tyystjärvi, E & Aro, E-M|title=The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity|journal=Proceedings of the National Academy of Sciences of the USA|volume=93| year=1996|pages=2213–2218}}</ref> The following year, laser pulse photoinhibition experiments done by Itzhak Ohad's group led to the suggestion that charge recombination reactions may be damaging because they can lead to production of singlet oxygen.<ref name="Keren et al 1997">{{cite journal|author = Keren N, Berg A, van Kan PJM, Levanon H & Ohad I|title= Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: The role of back electron flow |journal=Proceedings of the National Academy of Sciences of the USA|volume=94|year=1997|pages=1579–1584 | doi = 10.1073/pnas.94.4.1579 }}</ref> The molecular mechanism(s) of photoinhibition are constantly under discussion. The newest candidate is the [[manganese]] mechanism suggested 2005 by the group of Esa Tyystjärvi.<ref name="Hakala et al 2005">{{cite journal|author = Hakala M, Tuominen I, Keränen M, Tyystjärvi T & Tyystjärvi E|title= Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II|journal=Biochimica et Biophysica Acta - Bioenergetics|volume=1706|year=2005|pages=68–80|doi=10.1016/j.bbabio.2004.09.001}}</ref> A similar mechanism was suggested by the group of Norio Murata, also in 2005.<ref name="Ohnishi et al 2005">{{cite journal|author = Ohnishi N, Allakhverdiev SI,Takahashi S, Higashi S, Watanabe M, Nishiyama Y & Murata N|title= Two-Step Mechanism of Photodamage to Photosystem II: Step 1 Occurs at the Oxygen-Evolving Complex and Step 2 Occurs at the Photochemical Reaction Center| journal=Biochemistry|volume=44|year=2005|pages=8494–8499|doi= 10.1021/bi047518q}}</ref> |
The first measurements of photoinhibition were published in 1956 by Bessel Kok.<ref>{{cite journal|author = Kok B|title= On the inhibition of photosynthesis by intense light |journal=Biochimica et Biophysica Acta|volume=21|issue=2|year=1956|pages=234–244|doi=10.1016/0006-3002(56)90003-8}}</ref> Even in the very first studies it became obvious that plants have a repair mechanism that continuously repairs photoinhibitory damage. In 1966, Jones and Kok measured the action spectrum of photoinhibition and found that ultraviolet light is highly photoinhibitory.<ref name="Jones 1966">{{cite journal|author = Jones LW & Kok B|title= Photoinhibition of chloroplast reactions. I. Kinetics and action spectra |journal=Plant Physiology|volume=41|issue=6|year=1966|pages=1037–1043|url=http://www.plantphysiol.org/cgi/content/abstract/41/6/1037}}</ref> The visible-light part of the action spectrum was found to have a peak in the red-light region, suggesting that chlorophylls act as photoreceptors of photoinhibition. In the 1980s, photoinhibition became a popular topic in photosynthesis research, and the concept of a damaging reaction counteracted by a repair process was re-invented. Research was stimulated by a paper by Kyle, Ohad and Arntzen in 1984, showing that photoinhibition is accompanied by selective loss of a 32-kDa protein, later identified as the PSII reaction center protein D1.<ref name="Kyle 1984">{{cite journal|author = Kyle DJ, Ohad I, Arntzen CJ|title= Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes|journal=Proceedings of the National Academy of Sciences of the USA|volume=81|issue=13|year=1984|pages=4070–4074|url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC345370/pdf/pnas00614-0148.pdf}}</ref> The photosensitivity of PSII from which the oxygen evolving complex had been inactivated with chemical treatment was studied in the 1980s and early 1990s.<ref name = "Callahan et al 1986">{{cite journal|author = Callahan FE, Becker DW & Cheniae GM|title= Studies on the photoactivation of the water-oxidizing enzyme. II. Characterization of weak light photoinhibition of PSII and its light-induced recovery|journal=Plant Physiology|volume=82| year=1986|pages=261–269|url=http://www.plantphysiol.org/cgi/content/abstract/82/1/261}}</ref><ref>{{cite journal|author = Jegerschöld C, Virgin I & Styring S|title= Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction |journal=Biochemistry|volume=29|year=1990|pages=6179–6186}}</ref> A paper by Imre Vass and coworkers in 1992 described the acceptor-side mechanism of photoinhibition.<ref name = "Vass et al 1992">{{cite journal|author = Vass I, Styring S, Hundal T, Koivuniemi M, Aro E-M & Andersson B|title=Reversible and irreversible intermediates during photoinhibition of photosystem II: Stable reduced Q<sub>A</sub> species promote chlorophyll triplet formation|journal=Proceedings of the National Academy of Sciences of the USA|volume=89| year=1992|pages=1408–1412 |doi = 10.1073/pnas.89.4.1408}}</ref> Measurements of production of [[singlet oxygen]] by photoinhibited PSII provided further evidence for an acceptor-side-type mechanism.<ref name="Hideg et al 1998">{{cite journal|author = Hideg É, Kálai T, Hideg K & Vass I|title= Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves|journal=Biochemistry|volume=37|issue=33|year=1998|pages=11405–11411|doi=10.1021/bi972890+}}</ref> The concept of a repair cycle that continuously repairs photoinhibitory damage, evolved and was reviewed by Aro et al. in 1993.<ref name="Aro et al 1993">{{cite journal|author = Aro E-M, Virgin I & Andersson B|title= Photoinhibition of Photosystem II – inactivation, protein damage and turnover|journal=Biophysica et Biochimica Acta |volume=1143|issue=2|year=1993|pages=113–134}}</ref> Many details of the repair cycle, including the finding that the FtsH [[protease]] plays an important role in the degradation of the D1 protein, have been discovered since.<ref name="Bailey et al 2002">{{cite journal|author = Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C & Mann NH|title=A critical role for the Var2 FtsH homologue of ''Arabidopsis thaliana'' in the Photosystem II repair cycle in vivo|journal=Journal of Biological Chemistry|volume=277|issue=3|year=2002|pages=2006–2011|doi=10.1074/jbc.M105878200}}</ref> In 1996, a paper by Tyystjärvi and Aro showed that that the rate constant of photoinhibition is directly proportional to light intensity, a result that opposed the former assumption that photoinhibition is caused by the fraction of light energy that exceeds the maximum capability of photosynthesis.<ref name="Tyystjarvi and Aro 1996">{{cite journal|author = Tyystjärvi, E & Aro, E-M|title=The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity|journal=Proceedings of the National Academy of Sciences of the USA|volume=93| year=1996|pages=2213–2218|url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC39937/pdf/pnas01509-0488.pdf}}</ref> The following year, laser pulse photoinhibition experiments done by Itzhak Ohad's group led to the suggestion that charge recombination reactions may be damaging because they can lead to production of singlet oxygen.<ref name="Keren et al 1997">{{cite journal|author = Keren N, Berg A, van Kan PJM, Levanon H & Ohad I|title= Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: The role of back electron flow |journal=Proceedings of the National Academy of Sciences of the USA|volume=94|year=1997|pages=1579–1584 | doi = 10.1073/pnas.94.4.1579 }}</ref> The molecular mechanism(s) of photoinhibition are constantly under discussion. The newest candidate is the [[manganese]] mechanism suggested 2005 by the group of Esa Tyystjärvi.<ref name="Hakala et al 2005">{{cite journal|author = Hakala M, Tuominen I, Keränen M, Tyystjärvi T & Tyystjärvi E|title= Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II|journal=Biochimica et Biophysica Acta - Bioenergetics|volume=1706|year=2005|pages=68–80|doi=10.1016/j.bbabio.2004.09.001}}</ref> A similar mechanism was suggested by the group of Norio Murata, also in 2005.<ref name="Ohnishi et al 2005">{{cite journal|author = Ohnishi N, Allakhverdiev SI,Takahashi S, Higashi S, Watanabe M, Nishiyama Y & Murata N|title= Two-Step Mechanism of Photodamage to Photosystem II: Step 1 Occurs at the Oxygen-Evolving Complex and Step 2 Occurs at the Photochemical Reaction Center| journal=Biochemistry|volume=44|year=2005|pages=8494–8499|doi= 10.1021/bi047518q}}</ref> |
||
==What is inhibited== |
==What is inhibited== |
||
[[Image:PhotosystemII.PNG|right|thumb|Cyanobacteria photosystem II, dimer, PDB 2AXT]] |
[[Image:PhotosystemII.PNG|right|thumb|Cyanobacteria photosystem II, dimer, PDB 2AXT]] |
||
Photoinhibition occurs in all organisms capable of oxygenic photosynthesis, from [[vascular plants]] to [[cyanobacteria]].<ref name="Tyystjarvi et al 2002">{{cite journal|author=Tyystjärvi T, Tuominen I, Herranen M, Aro E-M, Tyystjärvi E|title=Action spectrum of ''psbA'' gene transcription is similar to that of photoinhibition in ''Synechocystis'' sp. PCC 6803|journal=FEBS Letters|volume=516|year=2002|pages=167–171}}</ref><ref name="Nishiyama et al 2005b">{{cite journal|author=Nishiyama Y, Allakhverdiev SI & Murata N|title=Inhibition of the repair of Photosystem II by oxidative stress in cyanobacteria|journal=Photosynthesis Research|volume=516|year=2002|pages=167–171}}</ref> In both plants and cyanobacteria, blue light causes photoinhibition more efficiently than other wavelengths of visible light, and all wavelengths of ultraviolet light are more efficient than wavelengths of visible light.<ref name="Tyystjarvi et al 2002" /> Photoinhibition is a series of reactions that inhibit different activities of PSII, but there is no consensus on what these steps are. The activity of the [[oxygen-evolving complex]] of PSII is often found to be lost before the rest of the reaction centre loses activity.<ref name="Hakala et al 2005" /><ref name="Ohnishi et al 2005" /><ref name="Tyystjarvi 2008" /> However, inhibition of PSII membranes under anaerobic conditions leads primarily to inhibition of electron transfer on the acceptor side of PSII.<ref name="Vass et al 1992" /> Ultraviolet light causes inhibition of the oxygen-evolving complex before the rest of PSII becomes inhibited. [[Photosystem I]] (PSI) is less susceptible to light-induced damage than PSII, but slow inhibition of this photosystem has been observed.<ref>{{cite journal|author = Sonoike K|title= Photoinhibition of photosystem I: Its physiological significance in the chilling sensitivity of plants|journal=Plant and Cell Physiology|volume=37|year=1996|pages=239–247}}</ref> Photoinhibition of PSI occurs in chilling-sensitive plants and the reaction depends on electron flow from PSII to PSI. |
Photoinhibition occurs in all organisms capable of oxygenic photosynthesis, from [[vascular plants]] to [[cyanobacteria]].<ref name="Tyystjarvi et al 2002">{{cite journal|author=Tyystjärvi T, Tuominen I, Herranen M, Aro E-M, Tyystjärvi E|title=Action spectrum of ''psbA'' gene transcription is similar to that of photoinhibition in ''Synechocystis'' sp. PCC 6803|journal=FEBS Letters|volume=516|year=2002|pages=167–171|doi=10.1016/S0014-5793(02)02537-1}}</ref><ref name="Nishiyama et al 2005b">{{cite journal|author=Nishiyama Y, Allakhverdiev SI & Murata N|title=Inhibition of the repair of Photosystem II by oxidative stress in cyanobacteria|journal=Photosynthesis Research|volume=516|year=2002|pages=167–171|doi=10.1007/s11120-004-6434-0}}</ref> In both plants and cyanobacteria, blue light causes photoinhibition more efficiently than other wavelengths of visible light, and all wavelengths of ultraviolet light are more efficient than wavelengths of visible light.<ref name="Tyystjarvi et al 2002" /> Photoinhibition is a series of reactions that inhibit different activities of PSII, but there is no consensus on what these steps are. The activity of the [[oxygen-evolving complex]] of PSII is often found to be lost before the rest of the reaction centre loses activity.<ref name="Hakala et al 2005" /><ref name="Ohnishi et al 2005" /><ref name="Tyystjarvi 2008" /> However, inhibition of PSII membranes under anaerobic conditions leads primarily to inhibition of electron transfer on the acceptor side of PSII.<ref name="Vass et al 1992" /> Ultraviolet light causes inhibition of the oxygen-evolving complex before the rest of PSII becomes inhibited. [[Photosystem I]] (PSI) is less susceptible to light-induced damage than PSII, but slow inhibition of this photosystem has been observed.<ref>{{cite journal|author = Sonoike K|title= Photoinhibition of photosystem I: Its physiological significance in the chilling sensitivity of plants|journal=Plant and Cell Physiology|volume=37|year=1996|pages=239–247}}</ref> Photoinhibition of PSI occurs in chilling-sensitive plants and the reaction depends on electron flow from PSII to PSI. |
||
===Quantum yield=== |
===Quantum yield=== |
||
Photosystem II is damaged by light irrespective of light intensity.<ref name="Tyystjarvi 2008" /> The apparent [[quantum yield]] of the damaging reaction in typical leaves of higher plants exposed to visible light, as well as in isolated [[thylakoid]] membrane preparations, is in the range of 10<sup>−8</sup> to 10<sup>−7</sup> and independent of the intensity of light.<ref name= "Eckert et al 1991">{{cite journal|author = Eckert HJ, Geiken B, Bernarding J, Napiwotzki A,Eichler HJ & Renger G|title= |
Photosystem II is damaged by light irrespective of light intensity.<ref name="Tyystjarvi 2008" /> The apparent [[quantum yield]] of the damaging reaction in typical leaves of higher plants exposed to visible light, as well as in isolated [[thylakoid]] membrane preparations, is in the range of 10<sup>−8</sup> to 10<sup>−7</sup> and independent of the intensity of light.<ref name= "Eckert et al 1991">{{cite journal|author = Eckert HJ, Geiken B, Bernarding J, Napiwotzki A,Eichler HJ & Renger G|title= Two sites of photoinhibition of the electron-transfer in oxygen evolving and Tris-treated PS-II membrane-fragments from spinach|journal=Photosynthesis Research|volume=27|year=1991|pages=97–108|doi=10.1007/BF00033249}}</ref><ref name="Tyystjarvi and Aro 1996" /> This means that one PSII complex is damaged for every 10-100 million photons that are intercepted. Therefore, photoinhibition occurs at all light intensities and the rate constant of photoinhibition is directly proportional to light intensity. Some measurements suggest that dim light causes damage more efficiently than strong light.<ref name="Keren et al 1997" /> |
||
==Molecular mechanism(s)== |
==Molecular mechanism(s)== |
||
| Line 19: | Line 27: | ||
===Donor-side photoinhibition=== |
===Donor-side photoinhibition=== |
||
If the oxygen-evolving complex is chemically inactivated, then the remaining electron transfer activity of PSII becomes very sensitive to light.<ref name="Eckert et al 1991" /><ref name = "Callahan et al 1986" /> It has been suggested that even in a healthy leaf, the oxygen-evolving complex does not always function in all PSII centers, and those ones are prone to rapid irreversible photoinhibition.<ref name="Anderson et al 1998">{{cite journal|author = Anderson JM, Park Y-I & Chow WS|title= Unifying model for the photoinactivation of Photosystem II ''in vivo'': a hypothesis |journal=Photosynthesis Research|volume=56|year=1998|pages=1–13}}</ref> |
If the oxygen-evolving complex is chemically inactivated, then the remaining electron transfer activity of PSII becomes very sensitive to light.<ref name="Eckert et al 1991" /><ref name = "Callahan et al 1986" /> It has been suggested that even in a healthy leaf, the oxygen-evolving complex does not always function in all PSII centers, and those ones are prone to rapid irreversible photoinhibition.<ref name="Anderson et al 1998">{{cite journal|author = Anderson JM, Park Y-I & Chow WS|title= Unifying model for the photoinactivation of Photosystem II ''in vivo'': a hypothesis |journal=Photosynthesis Research|volume=56|year=1998|pages=1–13|doi=10.1007/s004250000398}}</ref> |
||
===Manganese mechanism=== |
===Manganese mechanism=== |
||
| Line 25: | Line 33: | ||
===Singlet oxygen mechanisms=== |
===Singlet oxygen mechanisms=== |
||
Inhibition of PSII is caused by singlet oxygen produced either by weakly coupled chlorophyll molecules<ref name="Santabarbara et al 2002">{{cite journal|author = Santabarbara S, Cazzalini I, Rivadossi A, Garlaschi FM, Zucchelli G & Jennings RC|title= Photoinhibition ''in vivo'' and ''in vitro'' involves weakly coupled chlorophyll-protein complexes|journal=Photochemistry and Photobiology|volume=75|year=2002|pages=613–618}}</ref> or by [[cytochrome]]s or [[Iron-sulfur protein|iron-sulfur]] centers.<ref name="Jung and Kim 1990">{{cite journal|author = Jung J & Kim H-S|title= The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids|journal=Photochemistry and Photobiology|volume=52|year=1990|pages=1003–1009}}</ref> |
Inhibition of PSII is caused by singlet oxygen produced either by weakly coupled chlorophyll molecules<ref name="Santabarbara et al 2002">{{cite journal|author = Santabarbara S, Cazzalini I, Rivadossi A, Garlaschi FM, Zucchelli G & Jennings RC|title= Photoinhibition ''in vivo'' and ''in vitro'' involves weakly coupled chlorophyll-protein complexes|journal=Photochemistry and Photobiology|volume=75|year=2002|pages=613–618|doi=10.1562/0031-8655(2002)0750613PIVAIV2.0.CO2}}</ref> or by [[cytochrome]]s or [[Iron-sulfur protein|iron-sulfur]] centers.<ref name="Jung and Kim 1990">{{cite journal|author = Jung J & Kim H-S|title= The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids|journal=Photochemistry and Photobiology|volume=52|year=1990|pages=1003–1009|doi=10.1111/j.1751-1097.1990.tb01817.x}}</ref> |
||
===Low-light mechanism=== |
===Low-light mechanism=== |
||
| Line 31: | Line 39: | ||
==Kinetics and action spectrum== |
==Kinetics and action spectrum== |
||
Photoinhibition follows simple [[Rate_equation#First-order_reactions|first-order kinetics]] if measured from a [[lincomycin]]-treated leaf, cyanobacterial or algal cells, or from isolated thylakoid membranes in which concurrent repair does not disturb the kinetics. Data from the group of W. S. Chow indicate that in leaves of pepper (''[[Capsicum annuum]]''), the first-order pattern is replaced by a pseudo equilibrium even if the repair reaction is blocked. The deviation has been explained by assuming that photoinhibited PSII centers protect the remaining active ones.<ref>{{cite journal|author = Lee HY, Hong YN & Chow WS|title= Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in ''Capsicum annuum'' L. leaves|journal=Planta|volume=212|year=2001|pages=332–342}}</ref> |
Photoinhibition follows simple [[Rate_equation#First-order_reactions|first-order kinetics]] if measured from a [[lincomycin]]-treated leaf, cyanobacterial or algal cells, or from isolated thylakoid membranes in which concurrent repair does not disturb the kinetics. Data from the group of W. S. Chow indicate that in leaves of pepper (''[[Capsicum annuum]]''), the first-order pattern is replaced by a pseudo equilibrium even if the repair reaction is blocked. The deviation has been explained by assuming that photoinhibited PSII centers protect the remaining active ones.<ref>{{cite journal|author = Lee HY, Hong YN & Chow WS|title= Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in ''Capsicum annuum'' L. leaves|journal=Planta|volume=212|year=2001|pages=332–342|doi=10.1007/s004250000398}}</ref> |
||
Both visible and ultraviolet light cause photoinhibition, ultraviolet wavelengths being much more damaging.<ref name="Hakala et al 2005" /><ref name="Jung and Kim 1990" /><ref name="Sarvikas 2006">{{cite journal|author = Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T & Tyystjärvi E|title= Action spectrum of photoinhibition in leaves of wild type and ''npq1-2'' and ''npq4-1'' mutants of ''Arabidopsis thaliana''|journal=Plant and Cell Physiology|volume=47|year=2006|pages=391–400|doi=10.1093/pcp/pcj006}}</ref> Some researchers consider ultraviolet and visible light induced photoinhibition as a two different reactions<ref>{{cite journal|author = Sicora C, Mate Z & Vass I|title= The interaction of visible and UV-B light during photodamage and repair of Photosystem II|journal=Photosynthesis Research|volume=75|year=2003|pages=127–137}}</ref> while others stress the similarities between the inhibition reactions occurring under different wavelength ranges.<ref name="Hakala et al 2005" /><ref name="Ohnishi et al 2005" /> |
Both visible and ultraviolet light cause photoinhibition, ultraviolet wavelengths being much more damaging.<ref name="Hakala et al 2005" /><ref name="Jung and Kim 1990" /><ref name="Sarvikas 2006">{{cite journal|author = Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T & Tyystjärvi E|title= Action spectrum of photoinhibition in leaves of wild type and ''npq1-2'' and ''npq4-1'' mutants of ''Arabidopsis thaliana''|journal=Plant and Cell Physiology|volume=47|year=2006|pages=391–400|doi=10.1093/pcp/pcj006}}</ref> Some researchers consider ultraviolet and visible light induced photoinhibition as a two different reactions<ref>{{cite journal|author = Sicora C, Mate Z & Vass I|title= The interaction of visible and UV-B light during photodamage and repair of Photosystem II|journal=Photosynthesis Research|volume=75|year=2003|pages=127–137|doi=10.1023/A:1022852631339}}</ref> while others stress the similarities between the inhibition reactions occurring under different wavelength ranges.<ref name="Hakala et al 2005" /><ref name="Ohnishi et al 2005" /> |
||
==Reactive oxygen species== |
==Reactive oxygen species== |
||
| Line 55: | Line 63: | ||
==Dynamic photoinhibition== |
==Dynamic photoinhibition== |
||
Some researchers prefer to define the term “photoinhibition” so that it contains all reactions that lower the quantum yield of photosynthesis when a plant is exposed to light.<ref>{{cite journal|author = Powles SB|title= Photoinhibition of photosynthesis induced by visible light|journal=Annual Review of Plant Physiology and Plant Molecular Biology|volume=35|year=1984|pages=15–44}}</ref><ref>{{cite book|author=Hall DO & Rao KK|year=1999|title=Photosynthesis|publisher=Cambridge University Press, Cambridge}}</ref> In this case, the term "dynamic photoinhibition" comprises phenomena that reversibly down-regulate photosynthesis in the light and the term "photodamage" or "irreversible photoinhibition" covers the concept of photoinhibition used by other researchers. The main mechanism of dynamic photoinhibition is [[non-photochemical quenching]] of excitation energy absorbed by PSII. Dynamic photoinhibition is [[acclimation]] to strong light rather than light-induced damage, and therefore "dynamic photoinhibition" may actually protect the plant against "photoinhibition". |
Some researchers prefer to define the term “photoinhibition” so that it contains all reactions that lower the quantum yield of photosynthesis when a plant is exposed to light.<ref>{{cite journal|author = Powles SB|title= Photoinhibition of photosynthesis induced by visible light|journal=Annual Review of Plant Physiology and Plant Molecular Biology|volume=35|year=1984|pages=15–44|doi=10.1146/annurev.pp.35.060184.000311}}</ref><ref>{{cite book|author=Hall DO & Rao KK|year=1999|title=Photosynthesis|publisher=Cambridge University Press, Cambridge|isbn=9780521644976}}</ref> In this case, the term "dynamic photoinhibition" comprises phenomena that reversibly down-regulate photosynthesis in the light and the term "photodamage" or "irreversible photoinhibition" covers the concept of photoinhibition used by other researchers. The main mechanism of dynamic photoinhibition is [[non-photochemical quenching]] of excitation energy absorbed by PSII. Dynamic photoinhibition is [[acclimation]] to strong light rather than light-induced damage, and therefore "dynamic photoinhibition" may actually protect the plant against "photoinhibition". |
||
==Ecology of photoinhibition== |
==Ecology of photoinhibition== |
||
Revision as of 19:08, 1 April 2010

Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga or a cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also been used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light. Several recent reviews on photoinhibition are available.[1][2][3][4][5][6][7][8]
History
The first measurements of photoinhibition were published in 1956 by Bessel Kok.[9] Even in the very first studies it became obvious that plants have a repair mechanism that continuously repairs photoinhibitory damage. In 1966, Jones and Kok measured the action spectrum of photoinhibition and found that ultraviolet light is highly photoinhibitory.[10] The visible-light part of the action spectrum was found to have a peak in the red-light region, suggesting that chlorophylls act as photoreceptors of photoinhibition. In the 1980s, photoinhibition became a popular topic in photosynthesis research, and the concept of a damaging reaction counteracted by a repair process was re-invented. Research was stimulated by a paper by Kyle, Ohad and Arntzen in 1984, showing that photoinhibition is accompanied by selective loss of a 32-kDa protein, later identified as the PSII reaction center protein D1.[11] The photosensitivity of PSII from which the oxygen evolving complex had been inactivated with chemical treatment was studied in the 1980s and early 1990s.[12][13] A paper by Imre Vass and coworkers in 1992 described the acceptor-side mechanism of photoinhibition.[14] Measurements of production of singlet oxygen by photoinhibited PSII provided further evidence for an acceptor-side-type mechanism.[15] The concept of a repair cycle that continuously repairs photoinhibitory damage, evolved and was reviewed by Aro et al. in 1993.[16] Many details of the repair cycle, including the finding that the FtsH protease plays an important role in the degradation of the D1 protein, have been discovered since.[17] In 1996, a paper by Tyystjärvi and Aro showed that that the rate constant of photoinhibition is directly proportional to light intensity, a result that opposed the former assumption that photoinhibition is caused by the fraction of light energy that exceeds the maximum capability of photosynthesis.[18] The following year, laser pulse photoinhibition experiments done by Itzhak Ohad's group led to the suggestion that charge recombination reactions may be damaging because they can lead to production of singlet oxygen.[19] The molecular mechanism(s) of photoinhibition are constantly under discussion. The newest candidate is the manganese mechanism suggested 2005 by the group of Esa Tyystjärvi.[20] A similar mechanism was suggested by the group of Norio Murata, also in 2005.[21]
What is inhibited
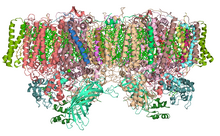
Photoinhibition occurs in all organisms capable of oxygenic photosynthesis, from vascular plants to cyanobacteria.[22][23] In both plants and cyanobacteria, blue light causes photoinhibition more efficiently than other wavelengths of visible light, and all wavelengths of ultraviolet light are more efficient than wavelengths of visible light.[22] Photoinhibition is a series of reactions that inhibit different activities of PSII, but there is no consensus on what these steps are. The activity of the oxygen-evolving complex of PSII is often found to be lost before the rest of the reaction centre loses activity.[20][21][2] However, inhibition of PSII membranes under anaerobic conditions leads primarily to inhibition of electron transfer on the acceptor side of PSII.[14] Ultraviolet light causes inhibition of the oxygen-evolving complex before the rest of PSII becomes inhibited. Photosystem I (PSI) is less susceptible to light-induced damage than PSII, but slow inhibition of this photosystem has been observed.[24] Photoinhibition of PSI occurs in chilling-sensitive plants and the reaction depends on electron flow from PSII to PSI.
Quantum yield
Photosystem II is damaged by light irrespective of light intensity.[2] The apparent quantum yield of the damaging reaction in typical leaves of higher plants exposed to visible light, as well as in isolated thylakoid membrane preparations, is in the range of 10−8 to 10−7 and independent of the intensity of light.[25][18] This means that one PSII complex is damaged for every 10-100 million photons that are intercepted. Therefore, photoinhibition occurs at all light intensities and the rate constant of photoinhibition is directly proportional to light intensity. Some measurements suggest that dim light causes damage more efficiently than strong light.[19]
Molecular mechanism(s)
The mechanism(s) of photoinhibition are under debate. The following mechanisms have been suggested:[2]
Acceptor-side photoinhibition
Strong light causes the reduction of the plastoquinone pool which leads to protonation and double reduction (and double protonation) of the QA electron acceptor of Photosystem II. The protonated and double reduced forms of QA do not function in electron transport. Furthermore, charge recombination reactions in inhibited Photosystem II are expected to lead to the triplet state of the primary donor (P680) more probably than same reactions in active PSII. Triplet P680 may react with oxygen to produce harmful singlet oxygen.[14]
Donor-side photoinhibition
If the oxygen-evolving complex is chemically inactivated, then the remaining electron transfer activity of PSII becomes very sensitive to light.[25][12] It has been suggested that even in a healthy leaf, the oxygen-evolving complex does not always function in all PSII centers, and those ones are prone to rapid irreversible photoinhibition.[26]
Manganese mechanism
A photon absorbed by the manganese ions of the oxygen-evolving complex triggers inactivation of the oxygen-evolving complex. Further inhibition of the remaining electron transport reactions occurs like in the donor-side mechanism. The mechanism is supported by the action spectrum of photoinhibition.[20]
Singlet oxygen mechanisms
Inhibition of PSII is caused by singlet oxygen produced either by weakly coupled chlorophyll molecules[27] or by cytochromes or iron-sulfur centers.[28]
Low-light mechanism
Charge recombination reactions of PSII cause the production of triplet P680 and consequently singlet oxygen. Charge recombination is more probable under dim light than under higher light intensities.[19]
Kinetics and action spectrum
Photoinhibition follows simple first-order kinetics if measured from a lincomycin-treated leaf, cyanobacterial or algal cells, or from isolated thylakoid membranes in which concurrent repair does not disturb the kinetics. Data from the group of W. S. Chow indicate that in leaves of pepper (Capsicum annuum), the first-order pattern is replaced by a pseudo equilibrium even if the repair reaction is blocked. The deviation has been explained by assuming that photoinhibited PSII centers protect the remaining active ones.[29] Both visible and ultraviolet light cause photoinhibition, ultraviolet wavelengths being much more damaging.[20][28][30] Some researchers consider ultraviolet and visible light induced photoinhibition as a two different reactions[31] while others stress the similarities between the inhibition reactions occurring under different wavelength ranges.[20][21]
Reactive oxygen species
Reactive oxygen species, especially singlet oxygen, have a role in the acceptor-side, singlet oxygen and low-light mechanisms. In the manganese mechanism and the donor side mechanism, reactive oxygen species do not have a direct role. Photoinhibited PSII produces singlet oxygen,[15] and reactive oxygen species inhibit the repair cycle of PSII by inhibiting translation elongation in the chloroplast. [5]
PSII repair cycle
Photoinhibition occurs continuously when plants or cyanobacteria are exposed to light, and the photosynthesizing organism must therefore continuously repair the damage.[16] The PSII repair cycle, occurring in chloroplasts and in cyanobacteria, consists of degradation and synthesis of the D1 protein of the PSII reaction centre, followed by activation of the reaction center. Due to the rapid repair, most PSII reaction centers are not photoinhibited even if a plant is grown in strong light. However environmental stresses, for example, extreme temperatures, salinity and drought, limit the supply of carbon dioxide for use in carbon fixation which decreases the rate of repair of PSII.[32]
In photoinhibition studies, repair is often stopped by applying an antibiotic (lincomycin or chloramphenicol) to plants or cyanobacteria which blocks protein synthesis in the chloroplast. Protein synthesis only occurs in an intact sample, so lincomycin is not needed when photoinhibition is measured from isolated membranes.[32] The repair cycle of PSII recirculates other subunits of PSII (except for the D1 protein) from the inhibited unit to the repaired one.
Protective mechanisms

Plants have mechanisms that protect against adverse effects of strong light. The most studied biochemical protective mechanism is non-photochemical quenching of excitation energy.[33] Visible-light-induced photoinhibition is ~25 % faster in an Arabidopsis thaliana mutant lacking non-photochemical quenching than in the wild type. It is also apparent that turning or folding of leaves, as occurs e.g. in Oxalis species in response to exposure to high light, protect against photoinhibition.
Measurement

Photoinhibition can be measured from isolated thylakoid membranes or their subfractions, or from intact cyanobacterial cells by measuring the light-saturated rate of oxygen evolution in the presence of an artificial electron acceptor (quinones and dichlorophenol-indophenol have been used). The degree of photoinhibition in intact leaves can be measured using a fluorimeter to measure the ratio of variable to maximum value of chlorophyll a fluorescence (FV/FM).[2] When measuring FV/FM, the leaf must be incubated in the dark for at least 10 minutes, preferably longer, before the measurement, in order to let non-photochemical quenching relax.
Flashing light
Photoinhibition can also be induced with short flashes of light using either a pulsed laser or a xenon flash lamp. When very short flashes are used, the photoinhibitory efficiency of the flashes depends on the time difference between the flashes.[19] This dependence has been interpreted to indicate that the flashes cause photoinhibition by inducing recombination reactions in PSII, with subsequent production of singlet oxygen. The interpretation has been criticized by noting that the photoinhibitory efficiency of xenon flashes depends on the energy of the flashes even if such strong flashes are used that they would saturate the formation of the substrate of the recombination reactions.[20]
Dynamic photoinhibition
Some researchers prefer to define the term “photoinhibition” so that it contains all reactions that lower the quantum yield of photosynthesis when a plant is exposed to light.[34][35] In this case, the term "dynamic photoinhibition" comprises phenomena that reversibly down-regulate photosynthesis in the light and the term "photodamage" or "irreversible photoinhibition" covers the concept of photoinhibition used by other researchers. The main mechanism of dynamic photoinhibition is non-photochemical quenching of excitation energy absorbed by PSII. Dynamic photoinhibition is acclimation to strong light rather than light-induced damage, and therefore "dynamic photoinhibition" may actually protect the plant against "photoinhibition".
Ecology of photoinhibition
Photoinhibition may cause coral bleaching.[32]
See also
References
- ^ Vass I & Cser K (2009). "Janus-faced charge recombinations in photosystem II photoinhibition". Trends in Plant Science. 14 (4): 200–205. doi:10.1016/j.tplants.2009.01.009.
- ^ a b c d e Tyystjärvi E (2008). "Photoinhibition of Photosystem II and photodamage of the oxygen-evolving manganese cluster". Coordination Chemistry Reviews. 252 (3–4): 361–376. doi:10.1016/j.ccr.2007.08.021.
- ^ Krieger-Liszkay A, Fufezan C & Trebst A (2008). "Singlet oxygen production in photosystem II and related protection mechanism". Photosynthesis Research. 98 (1–3): 551–564. doi:10.1007/s11120-008-9349-3.
- ^ Mohanty P, Allakhverdiev SI & Murata N (2007). "Application of low temperatures during photoinhibition allows characterization of individual steps in photodamage and the repair of photosystem II". Photosynthesis Research. 94 (2–3): 217–224. doi:10.1007/s11120-007-9184-y.
- ^ a b Nishiyama Y, Allakhverdiev SI & Murata N (2006). "A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II". Biophysica et Biochimica Acta - Bioenergetics. 1757 (7): 742–749. doi:10.1016/j.bbabio.2006.05.013.
- ^ Telfer A (2005). "Too much light? How beta-carotene protects the photosystem II reaction centre". Photochemical & Photobiological Sciences. 4 (12): 950–956. doi:10.1039/b507888c.
- ^
Adir N, Zer H, Shochat S & Ohad I (2003). "Photoinhibition - a historical perspective". Photosynthesis Research. 76 (1–3): 343–370. doi:10.1007/1-4020-3324-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Niyogi KK (1999). "Photoprotection revisited: Genetic and molecular approaches". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 333–359. doi:10.1146/annurev.arplant.50.1.333.
- ^ Kok B (1956). "On the inhibition of photosynthesis by intense light". Biochimica et Biophysica Acta. 21 (2): 234–244. doi:10.1016/0006-3002(56)90003-8.
- ^ Jones LW & Kok B (1966). "Photoinhibition of chloroplast reactions. I. Kinetics and action spectra". Plant Physiology. 41 (6): 1037–1043.
- ^ Kyle DJ, Ohad I, Arntzen CJ (1984). "Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes" (PDF). Proceedings of the National Academy of Sciences of the USA. 81 (13): 4070–4074.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Callahan FE, Becker DW & Cheniae GM (1986). "Studies on the photoactivation of the water-oxidizing enzyme. II. Characterization of weak light photoinhibition of PSII and its light-induced recovery". Plant Physiology. 82: 261–269.
- ^ Jegerschöld C, Virgin I & Styring S (1990). "Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction". Biochemistry. 29: 6179–6186.
- ^ a b c Vass I, Styring S, Hundal T, Koivuniemi M, Aro E-M & Andersson B (1992). "Reversible and irreversible intermediates during photoinhibition of photosystem II: Stable reduced QA species promote chlorophyll triplet formation". Proceedings of the National Academy of Sciences of the USA. 89: 1408–1412. doi:10.1073/pnas.89.4.1408.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Hideg É, Kálai T, Hideg K & Vass I (1998). "Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves". Biochemistry. 37 (33): 11405–11411. doi:10.1021/bi972890+.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Aro E-M, Virgin I & Andersson B (1993). "Photoinhibition of Photosystem II – inactivation, protein damage and turnover". Biophysica et Biochimica Acta. 1143 (2): 113–134.
- ^ Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C & Mann NH (2002). "A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the Photosystem II repair cycle in vivo". Journal of Biological Chemistry. 277 (3): 2006–2011. doi:10.1074/jbc.M105878200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Tyystjärvi, E & Aro, E-M (1996). "The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity" (PDF). Proceedings of the National Academy of Sciences of the USA. 93: 2213–2218.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Keren N, Berg A, van Kan PJM, Levanon H & Ohad I (1997). "Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: The role of back electron flow". Proceedings of the National Academy of Sciences of the USA. 94: 1579–1584. doi:10.1073/pnas.94.4.1579.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Hakala M, Tuominen I, Keränen M, Tyystjärvi T & Tyystjärvi E (2005). "Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II". Biochimica et Biophysica Acta - Bioenergetics. 1706: 68–80. doi:10.1016/j.bbabio.2004.09.001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Ohnishi N, Allakhverdiev SI,Takahashi S, Higashi S, Watanabe M, Nishiyama Y & Murata N (2005). "Two-Step Mechanism of Photodamage to Photosystem II: Step 1 Occurs at the Oxygen-Evolving Complex and Step 2 Occurs at the Photochemical Reaction Center". Biochemistry. 44: 8494–8499. doi:10.1021/bi047518q.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tyystjärvi T, Tuominen I, Herranen M, Aro E-M, Tyystjärvi E (2002). "Action spectrum of psbA gene transcription is similar to that of photoinhibition in Synechocystis sp. PCC 6803". FEBS Letters. 516: 167–171. doi:10.1016/S0014-5793(02)02537-1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nishiyama Y, Allakhverdiev SI & Murata N (2002). "Inhibition of the repair of Photosystem II by oxidative stress in cyanobacteria". Photosynthesis Research. 516: 167–171. doi:10.1007/s11120-004-6434-0.
- ^ Sonoike K (1996). "Photoinhibition of photosystem I: Its physiological significance in the chilling sensitivity of plants". Plant and Cell Physiology. 37: 239–247.
- ^ a b Eckert HJ, Geiken B, Bernarding J, Napiwotzki A,Eichler HJ & Renger G (1991). "Two sites of photoinhibition of the electron-transfer in oxygen evolving and Tris-treated PS-II membrane-fragments from spinach". Photosynthesis Research. 27: 97–108. doi:10.1007/BF00033249.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Anderson JM, Park Y-I & Chow WS (1998). "Unifying model for the photoinactivation of Photosystem II in vivo: a hypothesis". Photosynthesis Research. 56: 1–13. doi:10.1007/s004250000398.
- ^ Santabarbara S, Cazzalini I, Rivadossi A, Garlaschi FM, Zucchelli G & Jennings RC (2002). "Photoinhibition in vivo and in vitro involves weakly coupled chlorophyll-protein complexes". Photochemistry and Photobiology. 75: 613–618. doi:10.1562/0031-8655(2002)0750613PIVAIV2.0.CO2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jung J & Kim H-S (1990). "The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids". Photochemistry and Photobiology. 52: 1003–1009. doi:10.1111/j.1751-1097.1990.tb01817.x.
- ^ Lee HY, Hong YN & Chow WS (2001). "Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum L. leaves". Planta. 212: 332–342. doi:10.1007/s004250000398.
- ^ Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T & Tyystjärvi E (2006). "Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana". Plant and Cell Physiology. 47: 391–400. doi:10.1093/pcp/pcj006.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sicora C, Mate Z & Vass I (2003). "The interaction of visible and UV-B light during photodamage and repair of Photosystem II". Photosynthesis Research. 75: 127–137. doi:10.1023/A:1022852631339.
- ^ a b c Takahashi S & Murata N (2008). "How do environmental stresses accelerate photoinhibition". Trends in Plant Science. 13 (4): 178–182.
- ^ Krause GH & Jahns P (2004) "Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function" in Papageorgiou GC & Govindjee (eds.) "Chlorophyll Fluorescence: A Signature of Photosynthesis". pp. 463–495. Springer, The Netherlands. ISBN 978-1402032172
- ^ Powles SB (1984). "Photoinhibition of photosynthesis induced by visible light". Annual Review of Plant Physiology and Plant Molecular Biology. 35: 15–44. doi:10.1146/annurev.pp.35.060184.000311.
- ^ Hall DO & Rao KK (1999). Photosynthesis. Cambridge University Press, Cambridge. ISBN 9780521644976.
