Filbertone: Difference between revisions
Content deleted Content added
Filbertone is the principal flavor compound of hazelnuts... |
(No difference)
|
Revision as of 20:55, 9 November 2012
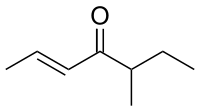
| |
| Names | |
|---|---|
| IUPAC name
(2E)-5-Methyl-2-hepten-4-one
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.133.148 |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H14O | |
| Molar mass | 126.199 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Filbertone is the principal flavor compound of hazelnuts.[1] It is used in perfumery and is designated as generally recognized as safe (GRAS) for use in foods.[2]
Because filbertone is found in hazelnut oil, its presence can be used to detect the adulteration of olive oil with less expensive hazelnut oil.[3][4]
The natural compound is mixture of both enantiomers, and the composition can vary depending on the source.[5][6]
References
- ^ . doi:10.1002/anie.198910221.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1590/S0103-50531998000600011.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1021/jf9807014.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/j.foodchem.2005.06.008.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1007/s11746-002-0527-1.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1007/BF01202621.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)
External links
- Filbertone, Molecule of the Month, University of Bristol
