Inotilone: Difference between revisions
Content deleted Content added
added Category:Phenols; removed {{uncategorized}} using HotCat |
chembox data |
||
| Line 3: | Line 3: | ||
| ImageSize = 150px |
| ImageSize = 150px |
||
| ImageAlt = |
| ImageAlt = |
||
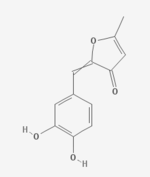
| IUPACName = 2-[(3,4-Dihydroxyphenyl)methylidene]-5-methyl-3-furanone |
|||
| IUPACName = |
|||
| OtherNames = |
| OtherNames = |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| CASNo = |
| CASNo = 906366-79-8 |
||
| PubChem = 53395281 |
| PubChem = 53395281 |
||
| InChI=1S/C12H10O4/c1-7-4-11(15)12(16-7)6-8-2-3-9(13)10(14)5-8/h2-6,13-14H,1H3 |
|||
| SMILES = }} |
|||
| InChIKey= NLZQGBCUKNUDED-UHFFFAOYSA-N |
|||
| SMILES = CC1=CC(=O)C(=CC2=CC(=C(C=C2)O)O)O1}} |
|||
| Section2 = {{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| C=12|H=10|O=4 |
|||
| Formula = |
|||
| MolarMass = |
|||
| Appearance = |
| Appearance = |
||
| Density = |
| Density = |
||
| Line 23: | Line 24: | ||
}} |
}} |
||
'''Inotilone''' is an anti-inflammatory isolate of ''[[Phellinus linteus]]''. |
'''Inotilone''' is an anti-inflammatory isolate of ''[[Phellinus linteus]]''.<ref>{{cite journal|pmid=22590514}}</ref> |
||
== |
==References== |
||
{{reflist}} |
|||
* [http://www.ncbi.nlm.nih.gov/pubmed/22590514 Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo] |
|||
| ⚫ | |||
| ⚫ | |||
[[Category:Phenols]] |
[[Category:Phenols]] |
||
Revision as of 11:03, 27 November 2013
| |
| Names | |
|---|---|
| IUPAC name
2-[(3,4-Dihydroxyphenyl)methylidene]-5-methyl-3-furanone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.116.430 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H10O4 | |
| Molar mass | 218.208 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Inotilone is an anti-inflammatory isolate of Phellinus linteus.[1]
References
