Somatic cell nuclear transfer: Difference between revisions
I added more information to the stem cell research. It needed more specific information regarding the most recent SCNT developments. My focus in particular was of the pioneering Tachibana procedure and tried to explain how they succeeded in deriving human |
|||
| Line 1: | Line 1: | ||
[[Image:Cloning diagram english.svg|thumb|300px|right|Somatic-cell nuclear transfer can create clones for both reproductive and therapeutic purposes. The diagram depicts the removal of the donor nucleus for schematic purposes; in practice usually the whole donor cell is transferred.]]In [[genetics]] and [[developmental biology]], '''somatic-cell nuclear transfer''' ('''SCNT''') is a [[laboratory technique]] for creating a |
[[Image:Cloning diagram english.svg|thumb|300px|right|Somatic-cell nuclear transfer can create clones for both reproductive and therapeutic purposes. The diagram depicts the removal of the donor nucleus for schematic purposes; in practice usually the whole donor cell is transferred.]]In [[genetics]] and [[developmental biology]], '''somatic-cell nuclear transfer''' ('''SCNT''') is a [[laboratory technique]] for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an enucleated oocyte (egg cell) and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. Dolly the Sheep, famous for being the first successfully cloned mammal was created using this process.<ref>{{cite pmid|19196043}}</ref> SCNT has been championed as an answer to the many issues concerning embryonic [[stem cell]]s (ESC) and the destruction of viable embryos in research. Though questions remain on how homologous the two cell types truly are. None-the-less SCNT is enthusiastically being utilized in stem cell research, with particular focus in aforementioned "'''therapeutic cloning'''", also know as [[regenerative medicine]]. The first step in the process of [[cloning|reproductive cloning]] is accomplished with SCNT. |
||
== The |
== The Process == |
||
{{Refimprove section|date=December 2011}} |
{{Refimprove section|date=December 2011}} |
||
The |
The process of somatic cell nuclear transplant involves two different cells. The first being a female gamete, know as the ovum (egg/oocyte). In human SCNT experiments, these eggs are obtained through consenting donors, many times utilizing ovarian stimulation. The second being a somatic cell, referring to the cells of the human body. Skin cells, fat cells, and liver cells are only a few examples. The nucleus of the donor egg cell is removed and discarded, leaving it 'deprogrammed.' The nucleus of the somatic cell is also removed but is kept, the enucleated somatic cell is discarded. What is left is a lone somatic nucleus and an enucleated egg cell. These are then fused by squirting the somatic nucleus into the 'empty' ovum. After being inserted into the egg, the somatic cell nucleus is [[Reprogramming|reprogrammed]] by its host egg cell. The ovum, now containing the somatic cell's nucleus, is stimulated with a shock and will begin to divide. The egg is now viable and capable of producing an adult organism containing all the necessary genetic information from just one parent. Development will ensue normally and after many mitotic divisions, this single cell forms a [[blastocyst]] (an early stage [[embryo]] with about 100 cells) with an identical genome to the original organism (i.e. a clone).<ref>{{cite pmid|9039911}}</ref> Stem cells can then be obtain by the destruction of this clone embryo for use in therapeutic cloning or in the case of reproductive cloning the clone embryo is implanted into a host mother for further development and brought to term. |
||
The technique of transferring a nucleus from a somatic cell into an egg that produced [[Dolly (sheep)|Dolly]] was an extension of experiments that had been ongoing for over 40 years. In the simplest terms, the technique used to produce Dolly the sheep – somatic-cell nuclear transplantation cloning – involves removing the nucleus of an egg and replacing it with the diploid nucleus of a somatic cell. |
|||
==Applications== |
|||
| ⚫ | |||
Some researchers use SCNT in [[stem cell research]]. The aim of carrying out this procedure is to obtain stem cells that are [[Genetics|genetically]] matched to the donor organism. |
|||
| ⚫ | |||
Embryonic stem cells are new, unspecialized cells that are able to be produced into a specialized cell that can replace another cell that has been lost in the body. |
|||
Somatic cell nuclear transplantation has become a focus of study in [[stem cell research]]. The aim of carrying out this procedure is to obtain pluripotent cells from a cloned embryo. These cells [[Genetics|genetically]] matched the donor organism from which they came.This gives them the ability to create patient specific pluripotent cells, which could then be used in therapies or disease research.<ref>{{cite pmid|24304071}}</ref> |
|||
Embryonic stem cells are undifferentiated cells of an embryo. These cells are deemed to have a pluripotent potential because the have the ability to give rise all of the tissues found in an adult organism. This ability allows stem cells to create any cell type, which could then be transplanted to replace damaged or destroyed cells. Controversy surrounds human ESC work due to the destruction of viable human embryos. Leading scientists to seek an alternative method of obtaining stem cells, SCNT is one such method. |
|||
[[Image:Human embryonic stem cell colony phase.jpg|left|thumb|220px|Human Embryonic Stem cell colony on mouse embryonic fibroblast feeder layer.]] |
[[Image:Human embryonic stem cell colony phase.jpg|left|thumb|220px|Human Embryonic Stem cell colony on mouse embryonic fibroblast feeder layer.]] |
||
A potential use of |
A potential use of stem cells genetically matched to a patient would be to create cell lines that have genes linked to a patient's particular disease. By doing so, an ''in vitro'' model could be created, would be useful for studying that particular disease, potentially discovering its pathophysiology, and discovering therapies.<ref>{{cite pmid|19366754}}</ref> For example, if a person with [[Parkinson's disease]] donated his or her somatic cells, the stem cells resulting from SCNT would have genes that contribute to Parkinson's disease. The disease specific stem cell lines could then be studied in order to better understand the condition.<ref name=Semb>{{cite journal |author=Semb H |title=Human embryonic stem cells: origin, properties and applications |journal=APMIS |volume=113 |issue=11–12 |pages=743–50 |year=2005 |pmid=16480446 |doi=10.1111/j.1600-0463.2005.apm_312.x |url=}}</ref> |
||
Another application of SCNT stem cell research is using the patient specific stem cell lines to generate tissues or even organs for transplant into the specific patient.<ref name="Pera">{{cite pmid|23765475}}</ref> The resulting cells would be genetically identical to the somatic-cell donor, thus avoiding any complications from [[transplant rejection|immune system rejection]].<ref name=Semb/><ref>{{cite journal |author=Hadjantonakis AK, Papaioannou VE |title=Can mammalian cloning combined with embryonic stem cell technologies be used to treat human diseases? |journal=Genome Biol. |volume=3 |issue=8 |pages=REVIEWS1023 |date=July 2002 |pmid=12186652 |pmc=139399 |doi= 10.1186/gb-2002-3-8-reviews1023|url=http://genomebiology.com/1465-6906/3/REVIEWS1023}}</ref> |
|||
Only a handful of the labs in the world are currently using SCNT techniques in human stem cell research. In the [[United States]], scientists at the [http://www.hsci.harvard.edu Harvard Stem Cell Institute], the [[University of California, San Francisco|University of California San Francisco]], the [[Oregon Health & Science University]],<ref name=Tachibana>{{cite journal |author=Tachibana M |title=Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer |journal=Cell |volume=In Press |year=2013 |doi=10.1016/j.cell.2013.05.006 |url=http://www.cell.com/abstract/S0092-8674(13)00571-0?switch=standard}}</ref> [http://www.stemagen.com Stemagen (La Jolla, CA)] and possibly [[Advanced Cell Technology]] are currently researching a technique to use somatic-cell nuclear transfer to produce [[embryonic stem cell]]s.<ref name="weise">Elizabeth Weise, "[http://www.usatoday.com/tech/science/genetics/2006-01-17-stem-cell-rejuvenated_x.htm Cloning race is on again]", ''USA Today'' (January 17, 2006, retrieved October 6, 2006)</ref> In the [[United Kingdom]], the [[Human Fertilisation and Embryology Authority]] has granted permission to research groups at the [[Roslin Institute]] and the [[Centre for Life|Newcastle Centre for Life]].<ref name="bbc">"[http://news.bbc.co.uk/2/hi/health/3695186.stm Dolly scientists' human clone bid]", ''BBC News'' (September 28, 2004, retrieved October 6, 2006)</ref> SCNT may also be occurring in China.<ref name="mann">Charles C. Mann, "[http://www.wired.com/wired/archive/11.01/cloning.html The First Cloning Superpower]", ''Wired'' (January 2003, retrieved October 6, 2006)</ref> |
Only a handful of the labs in the world are currently using SCNT techniques in human stem cell research. In the [[United States]], scientists at the [http://www.hsci.harvard.edu Harvard Stem Cell Institute], the [[University of California, San Francisco|University of California San Francisco]], the [[Oregon Health & Science University]],<ref name=Tachibana>{{cite journal |author=Tachibana M |title=Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer |journal=Cell |volume=In Press |year=2013 |doi=10.1016/j.cell.2013.05.006 |url=http://www.cell.com/abstract/S0092-8674(13)00571-0?switch=standard}}</ref> [http://www.stemagen.com Stemagen (La Jolla, CA)] and possibly [[Advanced Cell Technology]] are currently researching a technique to use somatic-cell nuclear transfer to produce [[embryonic stem cell]]s.<ref name="weise">Elizabeth Weise, "[http://www.usatoday.com/tech/science/genetics/2006-01-17-stem-cell-rejuvenated_x.htm Cloning race is on again]", ''USA Today'' (January 17, 2006, retrieved October 6, 2006)</ref> In the [[United Kingdom]], the [[Human Fertilisation and Embryology Authority]] has granted permission to research groups at the [[Roslin Institute]] and the [[Centre for Life|Newcastle Centre for Life]].<ref name="bbc">"[http://news.bbc.co.uk/2/hi/health/3695186.stm Dolly scientists' human clone bid]", ''BBC News'' (September 28, 2004, retrieved October 6, 2006)</ref> SCNT may also be occurring in China.<ref name="mann">Charles C. Mann, "[http://www.wired.com/wired/archive/11.01/cloning.html The First Cloning Superpower]", ''Wired'' (January 2003, retrieved October 6, 2006)</ref> |
||
| Line 20: | Line 21: | ||
In 2005, a [[South Korea]]n research team led by Professor [[Hwang Woo-suk]], published claims to have derived stem cell lines via SCNT,<ref>{{cite journal |author=Hwang WS, Roh SI, Lee BC, ''et al.'' |title=Patient-specific embryonic stem cells derived from human SCNT blastocysts |journal=Science |volume=308 |issue=5729 |pages=1777–83 |date=June 2005 |pmid=15905366 |doi=10.1126/science.1112286 |url=}}{{Retracted paper|intentional=yes}}</ref> but supported those claims with fabricated data.<ref>{{cite journal |author=Kennedy D |title=Editorial retraction |journal=Science |volume=311 |issue=5759 |pages=335 |date=January 2006 |pmid=16410485 |doi=10.1126/science.1124926 |url=}}</ref> Recent evidence has proved that he in fact created a stem cell line from a [[Parthenogenesis|parthenote]].<ref>[http://blogs.nature.com/reports/theniche/2007/06/hwangs_clone_was_really_a_part_2.html], Nature Stem Cell Blog.</ref><ref>[http://www.the-scientist.com/blog/display/53290/], The Scientist 19 June 2007</ref> |
In 2005, a [[South Korea]]n research team led by Professor [[Hwang Woo-suk]], published claims to have derived stem cell lines via SCNT,<ref>{{cite journal |author=Hwang WS, Roh SI, Lee BC, ''et al.'' |title=Patient-specific embryonic stem cells derived from human SCNT blastocysts |journal=Science |volume=308 |issue=5729 |pages=1777–83 |date=June 2005 |pmid=15905366 |doi=10.1126/science.1112286 |url=}}{{Retracted paper|intentional=yes}}</ref> but supported those claims with fabricated data.<ref>{{cite journal |author=Kennedy D |title=Editorial retraction |journal=Science |volume=311 |issue=5759 |pages=335 |date=January 2006 |pmid=16410485 |doi=10.1126/science.1124926 |url=}}</ref> Recent evidence has proved that he in fact created a stem cell line from a [[Parthenogenesis|parthenote]].<ref>[http://blogs.nature.com/reports/theniche/2007/06/hwangs_clone_was_really_a_part_2.html], Nature Stem Cell Blog.</ref><ref>[http://www.the-scientist.com/blog/display/53290/], The Scientist 19 June 2007</ref> |
||
Though there has been numerous successes with cloning animals, questions remain concerning the mechanisms of reprogramming in the ovum. Despite many attempts, success in creating human nuclear transfer embryonic stem cells has been limited. There lies a problem in the human cells ability to form a blastocyst; the cells fail to progress past the eight cell stage of development. This is thought to be a result from the somatic cell nucleus being unable to turn on embryonic genes crucial for proper development. These earlier experiments used procedures developed in non-primate animals with little success. A research group from the [[Oregon Health & Science University]] demonstrated SCNT procedures developed for primates successfully reprogrammed skin cells into stem cells. The key to their success was utilizing oocytes in metaphase II (MII) of the cell cycle. Egg cells in MII contain special factors in the cytoplasm that have a special ability in reprogramming implanted somatic cell nuclei into cells with pluripotent states. When the ovum's nucleus is removed, the cell loses its genetic information. This has been blamed for why enucleated eggs are hampered in their reprogramming ability. It is theorized the critical embryonic genes are physically linked to oocyte chromosomes, enucleation negatively affects these factors. Another possibility is removing the egg nucleus or inserting the somatic nucleus causes damage to the cytoplast, affecting reprogramming ability. Taking this into account the research group applied their new technique in an attempt to produce human SCNT stem cells. In May 2013, the Oregon group reported the successful derivation of human embryonic stem cell lines derived through SCNT, using fetal and infant donor cells. Using MII oocytes from volunteers and thier improved SCNT procedure, human clone embryos were successfully produced. These embryos were of poor quality, lacking a substantial inner cell mass and poorly constructed trophectoderm. The imperfect embryos prevented the acquisition of human ESC. The addition of caffeine during the removal of the ovum's nucleus and injection of the somatic nucleus improved blastocyst formation and ESC isolation. The ESC obtain were found to be capable of producing teratomas, expressed pluripotent transcription factors, and expressed a normal 46XX karyotype, indicating these SCNT were in fact ESC-like. <ref name="Tachibana"/> This was the first instance of successfully using SCNT to reprogram human somatic cells. This study used fetal and infantile somatic cells to produce their ESC. |
|||
| ⚫ | In |
||
| ⚫ | In April 2014, an international research team expanded on this break through. There remained the question of whether the same success could be accomplished using adult somatic cells. Epigenetic and age related changes were thought to possibly hinder an adult somatic cells ability to be reprogrammed. Implementing the procedure pioneered by the Oregon research group they indeed were able to grow stem cells generated by SCNT using adult cells from two donors, aged 35 and 75.Indicating age does not impede a cells ability to be reprogrammed<ref>[http://www.cell.com/cell-stem-cell/fulltext/S1934-5909%2814%2900137-4 Human Somatic Cell Nuclear Transfer Using Adult Cells] ''[[Cell Stem Cell]]''. Retrieved 18 April 2014</ref> <ref>Ariana Eunjung Cha (18 April 2014) [http://www.washingtonpost.com/national/health-science/cloning-advance-using-cells-from-human-adult-raises-ethical-questions/2014/04/17/33a58222-c663-11e3-bf7a-be01a9b69cf1_story.html Cloning advance using stem cells from human adult reopens ethical questions] ''Washington Post''. Retrieved 18 April 2014</ref> |
||
| ⚫ | The impetus for SCNT-based stem cell research has been decreased by the development and improvement of alternative methods of generating stem cells. Methods to reprogram normal body cells into [[Induced pluripotent stem cell|pluripotent stem cells]] were developed in humans in 2007. The following year, this method achieved a key goal of SCNT-based stem cell research: the derivation of pluripotent stem cell lines that have all genes linked to various diseases.<ref name="2008 breakthrough">{{cite journal |author=Gretchen Vogel|title=Breakthrough of the year: Reprogramming Cells|journal=Science |volume=322|issue=5909|pages=1766–1767|date=December 2008|doi=10.1126/science.322.5909.1766|url=http://www.sciencemag.org/cgi/content/full/322/5909/1766 |pmid=19095902}}</ref> Some scientists working on SCNT-based stem cell research have recently moved to the new methods of induced pluripotent stem cells. |
||
Late April 2014, the [[New York Stem Cell Foundation]] was successful in creating SCNT stem cells derived from adult somatic cells. One of these lines of stem cells was derived from the donor cells of a type 1 diabetic. The group was then able to successfully culture these stem cells and induce differentiation. When injected into mice, cells of all three of the germ layers successfully formed. The most significant of these cells, were those who expressed insulin and were capable of secreting the hormone.<ref>{{cite pmid|24776804}}</ref> These insulin producing cells could be used for replacement therapy in diabetics, demonstrating real SCNT stem cell therapeutic potential. |
|||
| ⚫ | |||
| ⚫ | The impetus for SCNT-based stem cell research has been decreased by the development and improvement of alternative methods of generating stem cells. Methods to reprogram normal body cells into [[Induced pluripotent stem cell|pluripotent stem cells]] were developed in humans in 2007. The following year, this method achieved a key goal of SCNT-based stem cell research: the derivation of pluripotent stem cell lines that have all genes linked to various diseases.<ref name="2008 breakthrough">{{cite journal |author=Gretchen Vogel|title=Breakthrough of the year: Reprogramming Cells|journal=Science |volume=322|issue=5909|pages=1766–1767|date=December 2008|doi=10.1126/science.322.5909.1766|url=http://www.sciencemag.org/cgi/content/full/322/5909/1766 |pmid=19095902}}</ref> Some scientists working on SCNT-based stem cell research have recently moved to the new methods of induced pluripotent stem cells. Though recent studies have put in question how similar iPS cells are to embryonic stem cells. Epigenetic memory in iPS affects the cell lineage it can differentiate into. For instance, a iPS cell derived from a blood cell will be more efficient at differentiating into blood cells, while it will be less efficient at creating a neuron. <ref>{{cite pmid|20644535}}</ref> This raises the question of how well iPS cells can mimic the gold standard ESC in experiments, as stem cells are defined as having the ability to differentiate into any cell type. SCNT stem cells do not pose such a problem and continue to remain relevant in stem cell studies. |
||
| ⚫ | |||
{{main|Cloning}} |
{{main|Cloning}} |
||
[[File:ECM 2001 Hybridoma System.jpg|62 KBpx|thumbnail|default|BTX ECM 2001 Electrofusion generator used for SCNT and Cloning applications]] |
[[File:ECM 2001 Hybridoma System.jpg|62 KBpx|thumbnail|default|BTX ECM 2001 Electrofusion generator used for SCNT and Cloning applications]] |
||
This technique is currently the basis for [[cloning]] animals (such as the famous [[Dolly the sheep]]),<ref name=Campbell>{{cite journal |author=Campbell KH, McWhir J, Ritchie WA, Wilmut I |title=Sheep cloned by nuclear transfer from a cultured cell line |journal=Nature |volume=380 |issue=6569 |pages=64–6 |date=March 1996 |pmid=8598906 |doi=10.1038/380064a0 |url=}}</ref> and in theory could be used to clone humans. |
This technique is currently the basis for [[cloning]] animals (such as the famous [[Dolly the sheep]]),<ref name=Campbell>{{cite journal |author=Campbell KH, McWhir J, Ritchie WA, Wilmut I |title=Sheep cloned by nuclear transfer from a cultured cell line |journal=Nature |volume=380 |issue=6569 |pages=64–6 |date=March 1996 |pmid=8598906 |doi=10.1038/380064a0 |url=}}</ref> and in theory could be used to clone humans. Using SCNT in reprosuctive cloning has proven difficult with limited success. High fetal and neonatal death make the process very inefficient. Resulting cloned offspring are also plagued with development and imprinting disorders in non-human species. For these reasons, along with superfluous moral and ethical objections, reproductive cloning in humans is proscribed.<ref>{{cite pmid|22795681}}</ref> Most researchers believe that in the foreseeable future it will not be possible to use the current cloning technique to produce a human clone that will develop to term. It remains a possibility, though critical adjustments will be required to overcome current limitations during early embryonic development in human SCNT.<ref>{{cite journal |author=Revel M |title=Research on animal cloning technologies and their implications in medical ethics: an update |journal=Med Law |volume=19 |issue=3 |pages=527–43 |year=2000 |pmid=11143888 |doi= |url=}}</ref><ref>{{cite journal |author=Rhind SM, Taylor JE, De Sousa PA, King TJ, McGarry M, Wilmut I |title=Human cloning: can it be made safe? |journal=Nat. Rev. Genet. |volume=4 |issue=11 |pages=855–64 |date=November 2003 |pmid=14634633 |doi=10.1038/nrg1205 |url=}}</ref> |
||
There is also the potential for treating diseases associated with mutations in mitochondrial DNA. Recent studies show SCNT of the nucleus of a body cell afflicted with one of these diseases into a healthy oocyte prevents the inheritance of the mitochondrial disease. This treatment does not involve cloning but would produce a child with three genetic parents. A father providing a sperm cell, one mother providing the egg nucleus and another mother providing the enucleated egg cell.<ref name="Pera"></ref> |
|||
| ⚫ | |||
| ⚫ | |||
Interspecies nuclear transfer (iSCNT) is a means of somatic-cell nuclear transfer used to facilitate the rescue of endangered species, or even to restore species after their extinction. The technique is similar to SCNT [[cloning]] which typically is between domestic animals and rodents, or where there is a ready supply of [[oocyte]]s and surrogate animals. However, the cloning of highly endangered or extinct species requires the use of an alternative method of cloning. Interspecies nuclear transfer utilizes a host and a donor of two different organisms that are closely related species and within the same genus. In 2000, [[Robert Lanza]] was able to produce a cloned fetus of a [[gaur]], ''Bos gaurus'', combining it successfully with a domestic cow, ''Bos taurus''.<ref>{{cite journal|last=Lanza|first=Robert P.|coauthors=Jose B. Cibelli, Francisca A. Diaz, Carlos T. Moraes,Peter W. Farin, Charlotte E. Farin, Carolyn J. Hammer, Michael D. West,and Philip Damiani|title=Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer|journal=Cloning|year=2000|volume=2|issue=2|pages=79-90|url=http://media.longnow.org/files/2/REVIVE/Cloning%20of%20an%20Endangered%20Species.pdf|accessdate=10 December 2013|doi=10.1089/152045500436104}}</ref> |
Interspecies nuclear transfer (iSCNT) is a means of somatic-cell nuclear transfer used to facilitate the rescue of endangered species, or even to restore species after their extinction. The technique is similar to SCNT [[cloning]] which typically is between domestic animals and rodents, or where there is a ready supply of [[oocyte]]s and surrogate animals. However, the cloning of highly endangered or extinct species requires the use of an alternative method of cloning. Interspecies nuclear transfer utilizes a host and a donor of two different organisms that are closely related species and within the same genus. In 2000, [[Robert Lanza]] was able to produce a cloned fetus of a [[gaur]], ''Bos gaurus'', combining it successfully with a domestic cow, ''Bos taurus''.<ref>{{cite journal|last=Lanza|first=Robert P.|coauthors=Jose B. Cibelli, Francisca A. Diaz, Carlos T. Moraes,Peter W. Farin, Charlotte E. Farin, Carolyn J. Hammer, Michael D. West,and Philip Damiani|title=Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer|journal=Cloning|year=2000|volume=2|issue=2|pages=79-90|url=http://media.longnow.org/files/2/REVIVE/Cloning%20of%20an%20Endangered%20Species.pdf|accessdate=10 December 2013|doi=10.1089/152045500436104}}</ref> |
||
Interspecies nuclear transfer evidence of the universality of the triggering mechanism of the cell nucleus reprogramming. For example, Gupta et al.,<ref>Gupta, M. K., Das, Z. C., Heo, Y. T.,et al., & Uhm, S. J. (2013). [http://online.liebertpub.com/doi/abs/10.1089/cell.2012.0074 Transgenic Chicken, Mice, Cattle, and Pig Embryos by Somatic Cell Nuclear Transfer into Pig Oocytes]. Cellular Reprogramming (Formerly" Cloning and Stem Cells"), 15(4), 322-328 DOI:10.1089/cell.2012.0074.</ref>explored the possibility of producing transgenic cloned embryos by interspecies somatic cell nuclear transfer (iSCNT) of cattle, mice, and chicken donor cells into enucleated pig oocytes. Moreover, NCSU23 medium, which was designed for in vitro culture of pig embryos, was able to support the in vitro development of cattle, mice, and chicken iSCNT embryos up to the [[blastocyst]] stage. Furthermore, ovine oocyte cytoplast may be used for remodeling and reprogramming of human somatic cells back to the embryonic stage.<ref>S. Morteza Hosseini, Mehdi Hajian, Mohsen Forouzanfar et al. and Mohammad H. Nasr-Esfahani (2012). [http://online.liebertpub.com/doi/abs/10.1089/cell.2011.0061 Enucleated Ovine Oocyte Supports Human Somatic Cells Reprogramming Back to the Embryonic Stage]. Cellular Reprogramming, 14(2): 155—163. {{PMID|22384929}}</ref> |
Interspecies nuclear transfer evidence of the universality of the triggering mechanism of the cell nucleus reprogramming. For example, Gupta et al.,<ref>Gupta, M. K., Das, Z. C., Heo, Y. T.,et al., & Uhm, S. J. (2013). [http://online.liebertpub.com/doi/abs/10.1089/cell.2012.0074 Transgenic Chicken, Mice, Cattle, and Pig Embryos by Somatic Cell Nuclear Transfer into Pig Oocytes]. Cellular Reprogramming (Formerly" Cloning and Stem Cells"), 15(4), 322-328 DOI:10.1089/cell.2012.0074.</ref>explored the possibility of producing transgenic cloned embryos by interspecies somatic cell nuclear transfer (iSCNT) of cattle, mice, and chicken donor cells into enucleated pig oocytes. Moreover, NCSU23 medium, which was designed for in vitro culture of pig embryos, was able to support the in vitro development of cattle, mice, and chicken iSCNT embryos up to the [[blastocyst]] stage. Furthermore, ovine oocyte cytoplast may be used for remodeling and reprogramming of human somatic cells back to the embryonic stage.<ref>S. Morteza Hosseini, Mehdi Hajian, Mohsen Forouzanfar et al. and Mohammad H. Nasr-Esfahani (2012). [http://online.liebertpub.com/doi/abs/10.1089/cell.2011.0061 Enucleated Ovine Oocyte Supports Human Somatic Cells Reprogramming Back to the Embryonic Stage]. Cellular Reprogramming, 14(2): 155—163. {{PMID|22384929}}</ref> |
||
==Limitations== |
==Limitations== |
||
Stresses placed on both the egg cell and the introduced nucleus are enormous, |
SCNT is incredibly inefficient. Stresses placed on both the egg cell and the introduced nucleus are enormous, resulting in a low percentage of successfully reprogrammed cells. For example, Dolly the sheep was born after 277 eggs were used for SCNT, which created 29 viable embryos. Only three of these embryos survived until birth, and only one survived to adulthood.<ref name=Campbell/> As the procedure currently cannot be automated, but has to be performed manually under a [[microscope]], SCNT is very resource intensive. The [[biochemistry]] involved in reprogramming the [[cellular differentiation|differentiated]] somatic cell nucleus and activating the recipient egg is also far from understood. |
||
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's [[mitochondria]] that contain their own [[mitochondrial DNA]] are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus. |
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's [[mitochondria]] that contain their own [[mitochondrial DNA]] are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus. This fact may also hamper the potential benefits of SCNT derived tissues/organs for therapy, as there may be a immunoresponse to the non-self mtDNA after transplant. |
||
== Controversy == |
== Controversy == |
||
Revision as of 20:01, 29 May 2014

In genetics and developmental biology, somatic-cell nuclear transfer (SCNT) is a laboratory technique for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an enucleated oocyte (egg cell) and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. Dolly the Sheep, famous for being the first successfully cloned mammal was created using this process.[1] SCNT has been championed as an answer to the many issues concerning embryonic stem cells (ESC) and the destruction of viable embryos in research. Though questions remain on how homologous the two cell types truly are. None-the-less SCNT is enthusiastically being utilized in stem cell research, with particular focus in aforementioned "therapeutic cloning", also know as regenerative medicine. The first step in the process of reproductive cloning is accomplished with SCNT.
The Process
This section needs additional citations for verification. (December 2011) |
The process of somatic cell nuclear transplant involves two different cells. The first being a female gamete, know as the ovum (egg/oocyte). In human SCNT experiments, these eggs are obtained through consenting donors, many times utilizing ovarian stimulation. The second being a somatic cell, referring to the cells of the human body. Skin cells, fat cells, and liver cells are only a few examples. The nucleus of the donor egg cell is removed and discarded, leaving it 'deprogrammed.' The nucleus of the somatic cell is also removed but is kept, the enucleated somatic cell is discarded. What is left is a lone somatic nucleus and an enucleated egg cell. These are then fused by squirting the somatic nucleus into the 'empty' ovum. After being inserted into the egg, the somatic cell nucleus is reprogrammed by its host egg cell. The ovum, now containing the somatic cell's nucleus, is stimulated with a shock and will begin to divide. The egg is now viable and capable of producing an adult organism containing all the necessary genetic information from just one parent. Development will ensue normally and after many mitotic divisions, this single cell forms a blastocyst (an early stage embryo with about 100 cells) with an identical genome to the original organism (i.e. a clone).[2] Stem cells can then be obtain by the destruction of this clone embryo for use in therapeutic cloning or in the case of reproductive cloning the clone embryo is implanted into a host mother for further development and brought to term.
Applications
SCNT in stem cell research
Somatic cell nuclear transplantation has become a focus of study in stem cell research. The aim of carrying out this procedure is to obtain pluripotent cells from a cloned embryo. These cells genetically matched the donor organism from which they came.This gives them the ability to create patient specific pluripotent cells, which could then be used in therapies or disease research.[3]
Embryonic stem cells are undifferentiated cells of an embryo. These cells are deemed to have a pluripotent potential because the have the ability to give rise all of the tissues found in an adult organism. This ability allows stem cells to create any cell type, which could then be transplanted to replace damaged or destroyed cells. Controversy surrounds human ESC work due to the destruction of viable human embryos. Leading scientists to seek an alternative method of obtaining stem cells, SCNT is one such method.

A potential use of stem cells genetically matched to a patient would be to create cell lines that have genes linked to a patient's particular disease. By doing so, an in vitro model could be created, would be useful for studying that particular disease, potentially discovering its pathophysiology, and discovering therapies.[4] For example, if a person with Parkinson's disease donated his or her somatic cells, the stem cells resulting from SCNT would have genes that contribute to Parkinson's disease. The disease specific stem cell lines could then be studied in order to better understand the condition.[5]
Another application of SCNT stem cell research is using the patient specific stem cell lines to generate tissues or even organs for transplant into the specific patient.[6] The resulting cells would be genetically identical to the somatic-cell donor, thus avoiding any complications from immune system rejection.[5][7]
Only a handful of the labs in the world are currently using SCNT techniques in human stem cell research. In the United States, scientists at the Harvard Stem Cell Institute, the University of California San Francisco, the Oregon Health & Science University,[8] Stemagen (La Jolla, CA) and possibly Advanced Cell Technology are currently researching a technique to use somatic-cell nuclear transfer to produce embryonic stem cells.[9] In the United Kingdom, the Human Fertilisation and Embryology Authority has granted permission to research groups at the Roslin Institute and the Newcastle Centre for Life.[10] SCNT may also be occurring in China.[11]
In 2005, a South Korean research team led by Professor Hwang Woo-suk, published claims to have derived stem cell lines via SCNT,[12] but supported those claims with fabricated data.[13] Recent evidence has proved that he in fact created a stem cell line from a parthenote.[14][15]
Though there has been numerous successes with cloning animals, questions remain concerning the mechanisms of reprogramming in the ovum. Despite many attempts, success in creating human nuclear transfer embryonic stem cells has been limited. There lies a problem in the human cells ability to form a blastocyst; the cells fail to progress past the eight cell stage of development. This is thought to be a result from the somatic cell nucleus being unable to turn on embryonic genes crucial for proper development. These earlier experiments used procedures developed in non-primate animals with little success. A research group from the Oregon Health & Science University demonstrated SCNT procedures developed for primates successfully reprogrammed skin cells into stem cells. The key to their success was utilizing oocytes in metaphase II (MII) of the cell cycle. Egg cells in MII contain special factors in the cytoplasm that have a special ability in reprogramming implanted somatic cell nuclei into cells with pluripotent states. When the ovum's nucleus is removed, the cell loses its genetic information. This has been blamed for why enucleated eggs are hampered in their reprogramming ability. It is theorized the critical embryonic genes are physically linked to oocyte chromosomes, enucleation negatively affects these factors. Another possibility is removing the egg nucleus or inserting the somatic nucleus causes damage to the cytoplast, affecting reprogramming ability. Taking this into account the research group applied their new technique in an attempt to produce human SCNT stem cells. In May 2013, the Oregon group reported the successful derivation of human embryonic stem cell lines derived through SCNT, using fetal and infant donor cells. Using MII oocytes from volunteers and thier improved SCNT procedure, human clone embryos were successfully produced. These embryos were of poor quality, lacking a substantial inner cell mass and poorly constructed trophectoderm. The imperfect embryos prevented the acquisition of human ESC. The addition of caffeine during the removal of the ovum's nucleus and injection of the somatic nucleus improved blastocyst formation and ESC isolation. The ESC obtain were found to be capable of producing teratomas, expressed pluripotent transcription factors, and expressed a normal 46XX karyotype, indicating these SCNT were in fact ESC-like. [8] This was the first instance of successfully using SCNT to reprogram human somatic cells. This study used fetal and infantile somatic cells to produce their ESC.
In April 2014, an international research team expanded on this break through. There remained the question of whether the same success could be accomplished using adult somatic cells. Epigenetic and age related changes were thought to possibly hinder an adult somatic cells ability to be reprogrammed. Implementing the procedure pioneered by the Oregon research group they indeed were able to grow stem cells generated by SCNT using adult cells from two donors, aged 35 and 75.Indicating age does not impede a cells ability to be reprogrammed[16] [17]
Late April 2014, the New York Stem Cell Foundation was successful in creating SCNT stem cells derived from adult somatic cells. One of these lines of stem cells was derived from the donor cells of a type 1 diabetic. The group was then able to successfully culture these stem cells and induce differentiation. When injected into mice, cells of all three of the germ layers successfully formed. The most significant of these cells, were those who expressed insulin and were capable of secreting the hormone.[18] These insulin producing cells could be used for replacement therapy in diabetics, demonstrating real SCNT stem cell therapeutic potential.
The impetus for SCNT-based stem cell research has been decreased by the development and improvement of alternative methods of generating stem cells. Methods to reprogram normal body cells into pluripotent stem cells were developed in humans in 2007. The following year, this method achieved a key goal of SCNT-based stem cell research: the derivation of pluripotent stem cell lines that have all genes linked to various diseases.[19] Some scientists working on SCNT-based stem cell research have recently moved to the new methods of induced pluripotent stem cells. Though recent studies have put in question how similar iPS cells are to embryonic stem cells. Epigenetic memory in iPS affects the cell lineage it can differentiate into. For instance, a iPS cell derived from a blood cell will be more efficient at differentiating into blood cells, while it will be less efficient at creating a neuron. [20] This raises the question of how well iPS cells can mimic the gold standard ESC in experiments, as stem cells are defined as having the ability to differentiate into any cell type. SCNT stem cells do not pose such a problem and continue to remain relevant in stem cell studies.
SCNT in Reproductive Cloning

This technique is currently the basis for cloning animals (such as the famous Dolly the sheep),[21] and in theory could be used to clone humans. Using SCNT in reprosuctive cloning has proven difficult with limited success. High fetal and neonatal death make the process very inefficient. Resulting cloned offspring are also plagued with development and imprinting disorders in non-human species. For these reasons, along with superfluous moral and ethical objections, reproductive cloning in humans is proscribed.[22] Most researchers believe that in the foreseeable future it will not be possible to use the current cloning technique to produce a human clone that will develop to term. It remains a possibility, though critical adjustments will be required to overcome current limitations during early embryonic development in human SCNT.[23][24]
There is also the potential for treating diseases associated with mutations in mitochondrial DNA. Recent studies show SCNT of the nucleus of a body cell afflicted with one of these diseases into a healthy oocyte prevents the inheritance of the mitochondrial disease. This treatment does not involve cloning but would produce a child with three genetic parents. A father providing a sperm cell, one mother providing the egg nucleus and another mother providing the enucleated egg cell.[6]
Interspecies Nuclear Transfer
Interspecies nuclear transfer (iSCNT) is a means of somatic-cell nuclear transfer used to facilitate the rescue of endangered species, or even to restore species after their extinction. The technique is similar to SCNT cloning which typically is between domestic animals and rodents, or where there is a ready supply of oocytes and surrogate animals. However, the cloning of highly endangered or extinct species requires the use of an alternative method of cloning. Interspecies nuclear transfer utilizes a host and a donor of two different organisms that are closely related species and within the same genus. In 2000, Robert Lanza was able to produce a cloned fetus of a gaur, Bos gaurus, combining it successfully with a domestic cow, Bos taurus.[25]
Interspecies nuclear transfer evidence of the universality of the triggering mechanism of the cell nucleus reprogramming. For example, Gupta et al.,[26]explored the possibility of producing transgenic cloned embryos by interspecies somatic cell nuclear transfer (iSCNT) of cattle, mice, and chicken donor cells into enucleated pig oocytes. Moreover, NCSU23 medium, which was designed for in vitro culture of pig embryos, was able to support the in vitro development of cattle, mice, and chicken iSCNT embryos up to the blastocyst stage. Furthermore, ovine oocyte cytoplast may be used for remodeling and reprogramming of human somatic cells back to the embryonic stage.[27]
Limitations
SCNT is incredibly inefficient. Stresses placed on both the egg cell and the introduced nucleus are enormous, resulting in a low percentage of successfully reprogrammed cells. For example, Dolly the sheep was born after 277 eggs were used for SCNT, which created 29 viable embryos. Only three of these embryos survived until birth, and only one survived to adulthood.[21] As the procedure currently cannot be automated, but has to be performed manually under a microscope, SCNT is very resource intensive. The biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg is also far from understood.
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial DNA are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus. This fact may also hamper the potential benefits of SCNT derived tissues/organs for therapy, as there may be a immunoresponse to the non-self mtDNA after transplant.
Controversy
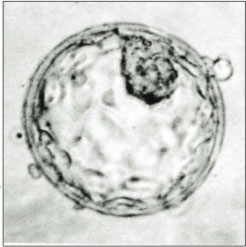
Proposals to use nucleus transfer techniques in human stem cell research raise a set of concerns beyond the moral status of any created embryo. These have led to some individuals and organizations who are not opposed to human embryonic stem cell research to be concerned about, or opposed to, SCNT research.[28][29][30]
One concern is that blastula creation in SCNT-based human stem cell research will lead to the reproductive cloning of humans. Both processes use the same first step: the creation of a nuclear transferred embryo, most likely via SCNT. Those who hold this concern often advocate for strong regulation of SCNT to preclude implantation of any derived products for the intention of human reproduction,[31] or its prohibition.[28]
A second important concern is the appropriate source of the eggs that are needed. SCNT requires human eggs, which can only be obtained from women. The most common source of these eggs today are eggs that are produced and in excess of the clinical need during IVF treatment. This is a minimally invasive procedure, but it does carry some health risks, such as ovarian hyperstimulation syndrome.
One vision for successful stem cell therapies is to create custom stem cell lines for patients. Each custom stem cell line would consist of a collection of identical stem cells each carrying the patient's own DNA, thus reducing or eliminating any problems with rejection when the stem cells were transplanted for treatment. For example, to treat a man with Parkinson's disease, a cell nucleus from one of his cells would be transplanted by SCNT into an egg cell from an egg donor, creating a unique lineage of stem cells almost identical to the patient's own cells. (There would be differences. For example, the mitochondrial DNA would be the same as that of the egg donor. In comparison, his own cells would carry the mitochondrial DNA of his mother.)
Potentially millions of patients could benefit from stem cell therapy, and each patient would require a large number of donated eggs in order to successfully create a single custom therapeutic stem cell line. Such large numbers of donated eggs would exceed the number of eggs currently left over and available from couples trying to have children through assisted reproductive technology. Therefore, healthy young women would need to be induced to sell eggs to be used in the creation of custom stem cell lines that could then be purchased by the medical industry and sold to patients. It is so far unclear where all these eggs would come from.
Stem cell experts consider it unlikely that such large numbers of human egg donations would occur in a developed country because of the unknown long-term public health effects of treating large numbers of healthy young women with heavy doses of hormones in order to induce hyperovulation (ovulating several eggs at once). Although such treatments have been performed for several decades now, the long-term effects have not been studied or declared safe to use on a large scale on otherwise healthy women. Longer-term treatments with much lower doses of hormones are known to increase the rate of cancer decades later. Whether hormone treatments to induce hyperovulation could have similar effects is unknown. There are also ethical questions surrounding paying for eggs. In general, marketing body parts is considered unethical and is banned in most countries. Human eggs have been a notable exception to this rule for some time.
To address the problem of creating a human egg market, some stem cell researchers are investigating the possibility of creating artificial eggs. If successful, human egg donations would not be needed to create custom stem cell lines. However, this technology may be a long way off.
Policies regarding human SCNT
SCNT involving human cells is currently legal for research purposes in the United Kingdom, having been incorporated into the Human Fertilisation and Embryology Act 1990 in 2001.[32] Permission must be obtained from the Human Fertilisation and Embryology Authority in order to perform or attempt SCNT.
In the United States, the practice remains legal, as it has not been addressed by federal law.[33] However, in 2002, a moratorium on United States federal funding for SCNT prohibits funding the practice for the purposes of research. Thus, though legal, SCNT cannot be federally funded.[34] American scholars have recently argued that because the product of SCNT is a clone embryo, rather than a human embryo, these policies are morally wrong and should be revised.[35]
In 2003, the United Nations adopted a proposal submitted by Costa Rica, calling on member states to "prohibit all forms of human cloning in as much as they are incompatible with human dignity and the protection of human life."[36] This phrase may include SCNT, depending on interpretation.
The Council of Europe's Convention on Human Rights and Biomedicine and its Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Being appear to ban SCNT of human beings. Of the Council's 45 member states, the Convention has been signed by 31 and ratified by 18. The Additional Protocol has been signed by 29 member nations and ratified by 14.[37]
The UN is currently against all forms of human cloning.[citation needed]
See also
- Induced stem cells
- Stem cell research
- Stem cell controversy
- Embryogenesis
- In vitro fertilisation
- Cloning
- New Jersey legislation S1909/A2840
- Rejuvenation
References
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19196043, please use {{cite journal}} with
|pmid=19196043instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9039911, please use {{cite journal}} with
|pmid=9039911instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 24304071, please use {{cite journal}} with
|pmid=24304071instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19366754, please use {{cite journal}} with
|pmid=19366754instead. - ^ a b Semb H (2005). "Human embryonic stem cells: origin, properties and applications". APMIS. 113 (11–12): 743–50. doi:10.1111/j.1600-0463.2005.apm_312.x. PMID 16480446.
- ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 23765475, please use {{cite journal}} with
|pmid=23765475instead. - ^ Hadjantonakis AK, Papaioannou VE (July 2002). "Can mammalian cloning combined with embryonic stem cell technologies be used to treat human diseases?". Genome Biol. 3 (8): REVIEWS1023. doi:10.1186/gb-2002-3-8-reviews1023. PMC 139399. PMID 12186652.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Tachibana M (2013). "Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer". Cell. In Press. doi:10.1016/j.cell.2013.05.006.
- ^ Elizabeth Weise, "Cloning race is on again", USA Today (January 17, 2006, retrieved October 6, 2006)
- ^ "Dolly scientists' human clone bid", BBC News (September 28, 2004, retrieved October 6, 2006)
- ^ Charles C. Mann, "The First Cloning Superpower", Wired (January 2003, retrieved October 6, 2006)
- ^ Hwang WS, Roh SI, Lee BC; et al. (June 2005). "Patient-specific embryonic stem cells derived from human SCNT blastocysts". Science. 308 (5729): 1777–83. doi:10.1126/science.1112286. PMID 15905366.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) (Retracted) - ^ Kennedy D (January 2006). "Editorial retraction". Science. 311 (5759): 335. doi:10.1126/science.1124926. PMID 16410485.
- ^ [1], Nature Stem Cell Blog.
- ^ [2], The Scientist 19 June 2007
- ^ Human Somatic Cell Nuclear Transfer Using Adult Cells Cell Stem Cell. Retrieved 18 April 2014
- ^ Ariana Eunjung Cha (18 April 2014) Cloning advance using stem cells from human adult reopens ethical questions Washington Post. Retrieved 18 April 2014
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 24776804, please use {{cite journal}} with
|pmid=24776804instead. - ^ Gretchen Vogel (December 2008). "Breakthrough of the year: Reprogramming Cells". Science. 322 (5909): 1766–1767. doi:10.1126/science.322.5909.1766. PMID 19095902.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20644535, please use {{cite journal}} with
|pmid=20644535instead. - ^ a b Campbell KH, McWhir J, Ritchie WA, Wilmut I (March 1996). "Sheep cloned by nuclear transfer from a cultured cell line". Nature. 380 (6569): 64–6. doi:10.1038/380064a0. PMID 8598906.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 22795681, please use {{cite journal}} with
|pmid=22795681instead. - ^ Revel M (2000). "Research on animal cloning technologies and their implications in medical ethics: an update". Med Law. 19 (3): 527–43. PMID 11143888.
- ^ Rhind SM, Taylor JE, De Sousa PA, King TJ, McGarry M, Wilmut I (November 2003). "Human cloning: can it be made safe?". Nat. Rev. Genet. 4 (11): 855–64. doi:10.1038/nrg1205. PMID 14634633.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lanza, Robert P. (2000). "Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer" (PDF). Cloning. 2 (2): 79–90. doi:10.1089/152045500436104. Retrieved 10 December 2013.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gupta, M. K., Das, Z. C., Heo, Y. T.,et al., & Uhm, S. J. (2013). Transgenic Chicken, Mice, Cattle, and Pig Embryos by Somatic Cell Nuclear Transfer into Pig Oocytes. Cellular Reprogramming (Formerly" Cloning and Stem Cells"), 15(4), 322-328 DOI:10.1089/cell.2012.0074.
- ^ S. Morteza Hosseini, Mehdi Hajian, Mohsen Forouzanfar et al. and Mohammad H. Nasr-Esfahani (2012). Enucleated Ovine Oocyte Supports Human Somatic Cells Reprogramming Back to the Embryonic Stage. Cellular Reprogramming, 14(2): 155—163. PMID 22384929
- ^ a b Jeremy Rifkin. (February 18, 2002). "Fusion Biopolitics". The Nation. Retrieved on August 7, 2006.
- ^ Sheryl Gay Stolberg, "Some for Abortion Rights Lean Right in Cloning Fight", New York Times (January 24, 2002)
- ^ Lori B. Andrews, et al., Open Letter to US Senate on Human Cloning, (March 19, 2002)
- ^ Lori B. Andrews et al. (March 19, 2002)."Open Letter to US Senators on Human Cloning and Eugenic Engineering". Retrieved on August 7, 2006
- ^ Andy Coghlan, "Cloning opponents fear loopholes in new UK law", New Scientist (November 23, 2001, retrieved October 6, 2006)
- ^ "Chapter 5: Legal and Policy Considerations. Cloning Human Beings" Report and Recommendations of the National Bioethics Advisory Commission, June 1997. Accessed 21 Oct 06
- ^ Robertson, John A. (2010). "Embryo Stem Cell Research: Ten Years of Controversy". The Journal of Law, Medicine, & Ethics, vol. 38. n. 2 pp. 191–203.
- ^ Cunningham, Thomas V. (2013). "What justifies the United States ban on federal funding for nonreproductive cloning?" Medicine, Health Care and Philosophy, vol. 16, n. 4, pp. 825-841.
- ^ United Nations, "General Assembly Adopts United Nations Declaration on Human Cloning By Vote of 84-34-37", press release (August 3, 2005, retrieved October 6, 2006)
- ^ Council of Europe, Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (April 4, 1997, retrieved October 6, 2006); Council of Europe, Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Being (January 12, 1998, retrieved October 6, 2006)
Further reading
- Wilmut I, Beaujean N, de Sousa PA; et al. (October 2002). "Somatic cell nuclear transfer". Nature. 419 (6907): 583–6. doi:10.1038/nature01079. PMID 12374931.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Kikyo N, Wolffe AP (January 2000). "Reprogramming nuclei: insights from cloning, nuclear transfer and heterokaryons". J. Cell. Sci. 113. ( Pt 1): 11–20. PMID 10591621.
- Tian XC, Kubota C, Enright B, Yang X (November 2003). "Cloning animals by somatic cell nuclear transfer—biological factors". Reprod. Biol. Endocrinol. 1 (1): 98. doi:10.1186/1477-7827-1-98. PMC 521203. PMID 14614770.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Gurdon JB, Byrne JA, Simonsson S (September 2003). "Nuclear reprogramming and stem cell creation". Proc. Natl. Acad. Sci. U.S.A. 100. Suppl 1 (90001): 11819–22. doi:10.1073/pnas.1834207100. PMC 304092. PMID 12920185.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Research Cloning: Medical and scientific, legal and ethical aspects
- The Basics: Stem Cells and Public Policy The Century Foundation, June 2005
- "Research Cloning Basic Science", Center for Genetics and Society, (Last modified October 4, 2004, retrieved October 6, 2006)
- Cloning: present uses and promises National Institutes of Health, Paper giving background information on cloning in general and SCNT from The Office of Science Policy Analysis.
- Nuclear Transfer – Stem Cells or Somatic Cell Nuclear Transfer (SCNT) The International Society for Stem Cell Research
- The Hinxton Group: An International Consortium on Stem Cells, Ethics & Law
