Finafloxacin: Difference between revisions
No edit summary |
Expanded. Removed stub tag. |
||
| Line 18: | Line 18: | ||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
||
| legal_status = |
| legal_status = |
||
| routes_of_administration = |
| routes_of_administration = otic, oral, intavenous |
||
<!-- Pharmacokinetic data --> |
<!-- Pharmacokinetic data --> |
||
| Line 25: | Line 25: | ||
| metabolism = |
| metabolism = |
||
| onset = |
| onset = |
||
| elimination_half-life = |
| elimination_half-life = 10 hours |
||
| excretion = |
| excretion = |
||
| Line 53: | Line 53: | ||
'''Finafloxacin''' ('''Xtoro''') is a [[fluoroquinolone antibiotic]]. In the United States, it is approved by the [[Food and Drug Administration]] to treat acute [[otitis externa]] (swimmer's ear) caused by the bacteria ''[[Pseudomonas aeruginosa]]'' and ''[[Staphylococcus aureus]]''.<ref>{{cite web | url =http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427274.htm | publisher = [[Food and Drug Administration]] | title = FDA approves Xtoro to treat swimmer’s ear | date = December 17, 2014}}</ref> |
'''Finafloxacin''' ('''Xtoro''') is a [[fluoroquinolone antibiotic]]. In the United States, it is approved by the [[Food and Drug Administration]] to treat acute [[otitis externa]] (swimmer's ear) caused by the bacteria ''[[Pseudomonas aeruginosa]]'' and ''[[Staphylococcus aureus]]''.<ref>{{cite web | url =http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427274.htm | publisher = [[Food and Drug Administration]] | title = FDA approves Xtoro to treat swimmer’s ear | date = December 17, 2014}}</ref> |
||
==Medical uses== |
|||
Finafloxacin is used to treat acute otitis externa caused by ''[[Pseudomonas aeruginosa]]'' and ''[[Staphylococcus aureus]]''.<ref name="APhA Review">{{cite web|title=Finafloxacin: New fluoroquinolone for acute otitis externa|url=https://www.pharmacist.com/finafloxacin-new-fluoroquinolone-acute-otitis-externa|website=pharmacist.com|publisher=American Pharmacists Association|accessdate=14 August 2017|date=February 1, 2015}}</ref> In the clinical trial that lead to the drug's approval, finafloxacin shortened the time to cessation of ear pain from an average of 6.8 days in patients taking a [[placebo]] to 3.5 days.<ref name="APhA Review" /> |
|||
===Available forms=== |
|||
Finafloxacin is commercially available as a 0.3% otic suspension for topical administration in the United States.<ref name="APhA Review" /> The suspension should be warmed gently in the hands for 1-2 minutes before administration to prevent dizziness, and shaken before use.<ref name="APhA Review" /> It is necessary to remain still for 1 minute, with the affected ear facing up while lying on one's side, after administration to allow finafloxacin to penetrate the ear canal and reach the site of infection.<ref name="APhA Review" /> |
|||
===Specific populations=== |
|||
Finafloxacin is classified as pregnancy category C.<ref name="PT Update">{{cite web|title=Rx Update: Xtoro (Finafloxacin Otic Suspension)|url=http://contemporaryclinic.pharmacytimes.com/journals/issue/2015/2015-vol1-n1/rx-update-xtoro-finafloxacin-otic-suspension|website=contemporaryclinic.pharmacytimes.com|publisher=Contemporary Clinic 2017 Pharmacy & Healthcare Communications, LLC|accessdate=14 August 2017}}</ref> |
|||
==Adverse effects== |
|||
The spectrum of adverse effects caused by finafloxacin vary by the method of administration. People that have administered finafloxacin into their ears in the form of drops have experienced ear [[itching]] and [[nausea]] (<1% for both).<ref name="McKeage Review" /> People that have administered finafloxacin by mouth or [[intravenously]] (IV) have experienced gastrointestinal side effects (including [[diarrhea]], [[flatulence]], and nausea), [[Fatigue (medical)|fatigue]], [[headaches]], musculoskeletal problems, and injection site reactions (if IV).<ref name="McKeage Review" /> Respiratory disorders, including [[rhinitis]] and [[nasopharyngitis]], have also been associated with the use of finafloxacin.<ref name="Kocsis et al Review" /> |
|||
People that are allergic to other quinolones may be allergic to finafloxacin as well, and use may result in an [[allergic reaction]] in that population.<ref name="APhA Review" /> |
|||
==Pharmacology== |
|||
===Mechanism of action=== |
|||
Finafloxacin is a [[fluoroquinolone]] class antibiotic of the 8-[[cyano]] subclass (referring to the CN substituent at the 8th position).<ref name="McKeage Review">{{cite journal|last1=McKeage|first1=Kate|title=Finafloxacin: First Global Approval|journal=Drugs|date=2015|volume=75|pages=687-693|doi=10.1007/s40265-015-0384-z|accessdate=13 August 2017}}</ref> Like other fluoroquinolones, its antibiotic activity is derived from its pharmacological mechanism of action as a [[Topoisomerase inhibitor|type II topoisomerase poison]], preventing bacteria from [[Cell division|replicating]] and performing other vital, [[Cell biology|cellular functions]].<ref name="McKeage Review" /> However, unlike other fluoroquinolones, finafloxacin is highly active under acidic ([[pH]] 5.0-6.0) conditions, where certain bacteria (like ''[[Helicobacter pylori]]'', a bacterium that is known to infect the human [[stomach]], despite the harsh acidity)<ref>{{cite journal|author=Blaser MJ|title=Who are we? Indigenous microbes and the ecology of human diseases|journal=EMBO Reports|volume=7|issue=10|pages=956–60|year=2006|pmid=17016449|pmc=1618379|doi=10.1038/sj.embor.7400812|url=http://www.nature.com/embor/journal/v7/n10/pdf/7400812.pdf}}</ref> thrive.<ref name="McKeage Review" /> Other acidic conditions found on the human body include the vagina, urinary tract, and skin, though finafloxacin is currently not used to treat infections in these areas either.<ref name="Kocsis et al Review" /> |
|||
Finafloxacin has demonstrated [[bactericidal]] activity against a range of bacterial pathogens, especially at acidic pH, with a [[Antimicrobial_pharmacodynamics#Post_Antibiotic_Effect|post-antibiotic effect]].<ref name="McKeage Review" /> Due to its activity against both [[Gram-positive]] and [[Gram-negative]] bacteria, finafloxacin is classified as a [[broad-spectrum antibiotic]].<ref name="McKeage Review" /> |
|||
===Pharmacokinetics=== |
|||
Finafloxacin has good oral [[bioavailability]], meaning that a substantial potion of a dose taken by mouth reaches a person's systemic circulation.<ref name="McKeage Review" /> Some people have experienced unintentional, quantifiable absorption of finafloxacin after administering the drug [[Ear drop|via the ear]].<ref name="McKeage Review" /> |
|||
The elimination half-life of finafloxacin is approximately 10 hours in humans.<ref name="Kocsis et al Review">{{cite journal|last1=Kocsis|first1=B|last2=Domokos|first2=J|last3=Szabo|first3=D|title=Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin|journal=Ann Clin Microbiol Antimicrob|date=2016|volume=15|pages=34|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4878067/|accessdate=13 August 2017}}</ref> |
|||
==Chemistry== |
|||
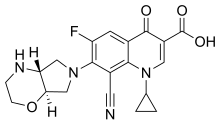
The chemical structure of finafloxacin has been described as a "fluorinated [[quinolone]] derivative with 8-[[cyano]]-substituent and 7-pyrrolo-oxazinyl moiety."<ref name="Kocsis et al Review" /> Its low isoelectric point (pH 6.7) is lower than the isoelectric point of another fluoroquinolone class antibiotic called [[ciprofloxacin]] (pH 7.4), which accounts for finafloxacin's superior activity at low pH (5.0-6.0).<ref name="Kocsis et al Review" /> |
|||
===Synthesis=== |
|||
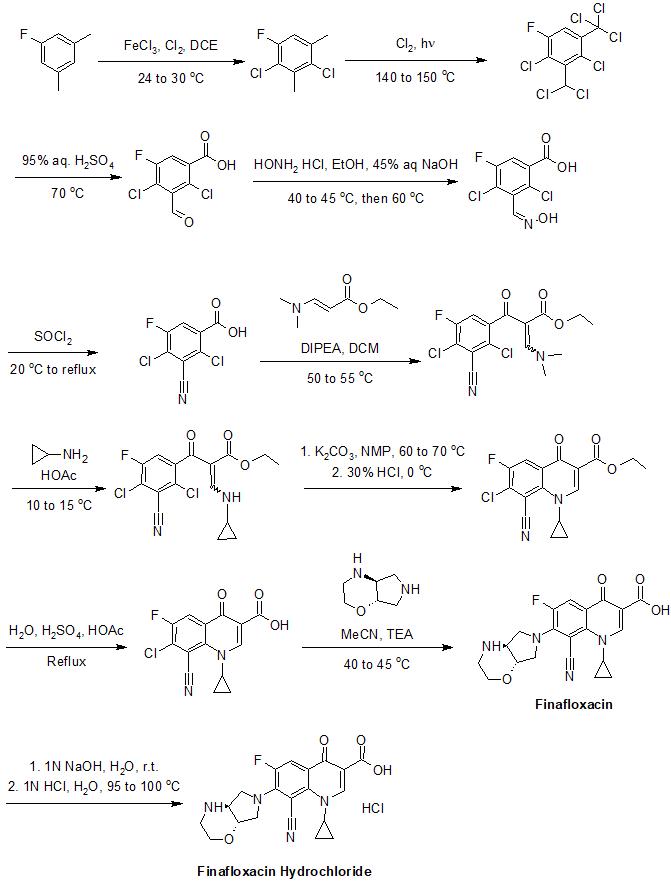
The synthesis of finafloxacin has been described in detail in its patents.<ref name="Pharmacodia">{{cite web|title=Finafloxacin|url=https://www.pharmacodia.com/yaodu/html/v1/chemicals/fd1d83de2517a02d4e221ede9a681432.html|website=pharmacodia.com|accessdate=14 August 2017}}</ref> An example of its synthesis is provided below:<ref name="Pharmacodia" /> |
|||
[[File:FinafloxacinSynthesis.jpg|Organic synthesis of finafloxacin hydrochloride.]] |
|||
==Research== |
|||
Due to its high bactericidal activity in acidic environments, some have speculated that finafloxacin may be useful in the treatment of [[urinary tract infections]] in the future.<ref name="Bartoletti et al Review UTI">{{cite journal|last1=Bartoletti|first1=R|last2=Cai|first2=T|last3=Perletti|first3=G|last4=Wagenlehner|first4=FME|last5=Bjerklund Johansen|first5=TE|title=Finafloxacin for the treatment of urinary tract infections|journal=Expert Opin Investig Drugs|date=2015|volume=24|issue=7|pages=957-63|doi=10.1517/13543784.2015.1052401|url=http://www.tandfonline.com/doi/abs/10.1517/13543784.2015.1052401?journalCode=ieid20|accessdate=14 August 2017}}</ref> |
|||
==References== |
==References== |
||
| Line 58: | Line 91: | ||
[[Category:Fluoroquinolone antibiotics]] |
[[Category:Fluoroquinolone antibiotics]] |
||
{{antibiotic-stub}} |
|||
Revision as of 00:00, 15 August 2017
 | |
| Clinical data | |
|---|---|
| Trade names | Xtoro |
| Routes of administration | otic, oral, intavenous |
| Pharmacokinetic data | |
| Elimination half-life | 10 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H19FN4O4 |
| Molar mass | 398.39 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Finafloxacin (Xtoro) is a fluoroquinolone antibiotic. In the United States, it is approved by the Food and Drug Administration to treat acute otitis externa (swimmer's ear) caused by the bacteria Pseudomonas aeruginosa and Staphylococcus aureus.[1]
Medical uses
Finafloxacin is used to treat acute otitis externa caused by Pseudomonas aeruginosa and Staphylococcus aureus.[2] In the clinical trial that lead to the drug's approval, finafloxacin shortened the time to cessation of ear pain from an average of 6.8 days in patients taking a placebo to 3.5 days.[2]
Available forms
Finafloxacin is commercially available as a 0.3% otic suspension for topical administration in the United States.[2] The suspension should be warmed gently in the hands for 1-2 minutes before administration to prevent dizziness, and shaken before use.[2] It is necessary to remain still for 1 minute, with the affected ear facing up while lying on one's side, after administration to allow finafloxacin to penetrate the ear canal and reach the site of infection.[2]
Specific populations
Finafloxacin is classified as pregnancy category C.[3]
Adverse effects
The spectrum of adverse effects caused by finafloxacin vary by the method of administration. People that have administered finafloxacin into their ears in the form of drops have experienced ear itching and nausea (<1% for both).[4] People that have administered finafloxacin by mouth or intravenously (IV) have experienced gastrointestinal side effects (including diarrhea, flatulence, and nausea), fatigue, headaches, musculoskeletal problems, and injection site reactions (if IV).[4] Respiratory disorders, including rhinitis and nasopharyngitis, have also been associated with the use of finafloxacin.[5]
People that are allergic to other quinolones may be allergic to finafloxacin as well, and use may result in an allergic reaction in that population.[2]
Pharmacology
Mechanism of action
Finafloxacin is a fluoroquinolone class antibiotic of the 8-cyano subclass (referring to the CN substituent at the 8th position).[4] Like other fluoroquinolones, its antibiotic activity is derived from its pharmacological mechanism of action as a type II topoisomerase poison, preventing bacteria from replicating and performing other vital, cellular functions.[4] However, unlike other fluoroquinolones, finafloxacin is highly active under acidic (pH 5.0-6.0) conditions, where certain bacteria (like Helicobacter pylori, a bacterium that is known to infect the human stomach, despite the harsh acidity)[6] thrive.[4] Other acidic conditions found on the human body include the vagina, urinary tract, and skin, though finafloxacin is currently not used to treat infections in these areas either.[5]
Finafloxacin has demonstrated bactericidal activity against a range of bacterial pathogens, especially at acidic pH, with a post-antibiotic effect.[4] Due to its activity against both Gram-positive and Gram-negative bacteria, finafloxacin is classified as a broad-spectrum antibiotic.[4]
Pharmacokinetics
Finafloxacin has good oral bioavailability, meaning that a substantial potion of a dose taken by mouth reaches a person's systemic circulation.[4] Some people have experienced unintentional, quantifiable absorption of finafloxacin after administering the drug via the ear.[4]
The elimination half-life of finafloxacin is approximately 10 hours in humans.[5]
Chemistry
The chemical structure of finafloxacin has been described as a "fluorinated quinolone derivative with 8-cyano-substituent and 7-pyrrolo-oxazinyl moiety."[5] Its low isoelectric point (pH 6.7) is lower than the isoelectric point of another fluoroquinolone class antibiotic called ciprofloxacin (pH 7.4), which accounts for finafloxacin's superior activity at low pH (5.0-6.0).[5]
Synthesis
The synthesis of finafloxacin has been described in detail in its patents.[7] An example of its synthesis is provided below:[7]
Research
Due to its high bactericidal activity in acidic environments, some have speculated that finafloxacin may be useful in the treatment of urinary tract infections in the future.[8]
References
- ^ "FDA approves Xtoro to treat swimmer's ear". Food and Drug Administration. December 17, 2014.
- ^ a b c d e f "Finafloxacin: New fluoroquinolone for acute otitis externa". pharmacist.com. American Pharmacists Association. February 1, 2015. Retrieved 14 August 2017.
- ^ "Rx Update: Xtoro (Finafloxacin Otic Suspension)". contemporaryclinic.pharmacytimes.com. Contemporary Clinic 2017 Pharmacy & Healthcare Communications, LLC. Retrieved 14 August 2017.
- ^ a b c d e f g h i McKeage, Kate (2015). "Finafloxacin: First Global Approval". Drugs. 75: 687–693. doi:10.1007/s40265-015-0384-z.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b c d e Kocsis, B; Domokos, J; Szabo, D (2016). "Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin". Ann Clin Microbiol Antimicrob. 15: 34. Retrieved 13 August 2017.
- ^ Blaser MJ (2006). "Who are we? Indigenous microbes and the ecology of human diseases" (PDF). EMBO Reports. 7 (10): 956–60. doi:10.1038/sj.embor.7400812. PMC 1618379. PMID 17016449.
- ^ a b "Finafloxacin". pharmacodia.com. Retrieved 14 August 2017.
- ^ Bartoletti, R; Cai, T; Perletti, G; Wagenlehner, FME; Bjerklund Johansen, TE (2015). "Finafloxacin for the treatment of urinary tract infections". Expert Opin Investig Drugs. 24 (7): 957–63. doi:10.1517/13543784.2015.1052401. Retrieved 14 August 2017.
