Isoguanine: Difference between revisions
Appearance
Content deleted Content added
m clean up using AWB |
mention role in hachimoji nucleic acids |
||
| Line 30: | Line 30: | ||
'''Isoguanine''' or '''2-hydroxyadenine''' is a [[purine]] base that is an [[isomer]] of [[guanine]]. It is a product of oxidative damage to DNA and has been shown to cause mutation.<ref>{{cite journal |vauthors=Yang XL, Sugiyama H, Ikeda S, Saito I, Wang AH |title=Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy |journal=Biophys. J. |volume=75 |issue=3 |pages=1163–1171 |year=1998 |pmid=9726918 |doi=10.1016/S0006-3495(98)74035-4 |pmc=1299791}}</ref> It is also used in combination with [[isocytosine]] in studies of unnatural [[nucleic acid analogues]] of the normal [[base pair]]s in DNA.<ref>Andrzej Jaworski, Józef S. Kwiatkowski, Bogdan Lesyng: „Why isoguanine and isocytosine are not the components of the genetic code", International Journal of Quantum Chemistry, Supplement: Proceedings of the International Symposium on Quantum Biology and Quantum Pharmacology, '''1985''', ''28'' (Supplement S12), pp. 209–216 ({{DOI|10.1002/qua.560280720}}).</ref><ref>Christopher Roberts, Rajanikanth Bandaru, Christopher Switzer: „Theoretical and Experimental Study of Isoguanine and Isocytosine: Base Pairing in an Expanded Genetic System", ''[[J. Am. Chem. Soc.]]'', '''1997''', ''119'' (20), pp. 4640–4649 ({{DOI|10.1021/ja970123s}}).</ref> |
'''Isoguanine''' or '''2-hydroxyadenine''' is a [[purine]] base that is an [[isomer]] of [[guanine]]. It is a product of oxidative damage to DNA and has been shown to cause mutation.<ref>{{cite journal |vauthors=Yang XL, Sugiyama H, Ikeda S, Saito I, Wang AH |title=Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy |journal=Biophys. J. |volume=75 |issue=3 |pages=1163–1171 |year=1998 |pmid=9726918 |doi=10.1016/S0006-3495(98)74035-4 |pmc=1299791}}</ref> It is also used in combination with [[isocytosine]] in studies of unnatural [[nucleic acid analogues]] of the normal [[base pair]]s in DNA.<ref>Andrzej Jaworski, Józef S. Kwiatkowski, Bogdan Lesyng: „Why isoguanine and isocytosine are not the components of the genetic code", International Journal of Quantum Chemistry, Supplement: Proceedings of the International Symposium on Quantum Biology and Quantum Pharmacology, '''1985''', ''28'' (Supplement S12), pp. 209–216 ({{DOI|10.1002/qua.560280720}}).</ref><ref>Christopher Roberts, Rajanikanth Bandaru, Christopher Switzer: „Theoretical and Experimental Study of Isoguanine and Isocytosine: Base Pairing in an Expanded Genetic System", ''[[J. Am. Chem. Soc.]]'', '''1997''', ''119'' (20), pp. 4640–4649 ({{DOI|10.1021/ja970123s}}).</ref> |
||
It is used as a [[nucleobase]] of [[hachimoji DNA|hachimoji nucleic acids]].<ref name="SCI-20190222">{{cite journal|last=Hoshika|first=Shuichi|display-authors=etal|date=22 February 2019|title=Hachimoji DNA and RNA: A genetic system with eight building blocks |journal=[[Science (journal)|Science]]|volume=363|issue=6429|pages=884-887|doi=10.1126/science.aat0971|subscription=yes}}</ref> In hachimoji DNA, it [[base pair|pairs with]] [[1-methylcytosine]], while in hachimoji RNA, it pairs with isocytosine. |
|||
[[File:IG-iC DNA base pair.svg|thumb|none|upright=1.6|Isoguanine-Isocytosine-base-pair]] |
[[File:IG-iC DNA base pair.svg|thumb|none|upright=1.6|Isoguanine-Isocytosine-base-pair]] |
||
Revision as of 10:46, 26 February 2019
| |
| Names | |
|---|---|
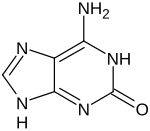
| IUPAC name
6-Amino-1,7-dihydropurin-2-one
| |
| Other names
2-Hydroxyadenine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.020.144 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H5N5O | |
| Molar mass | 151.1261 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isoguanine or 2-hydroxyadenine is a purine base that is an isomer of guanine. It is a product of oxidative damage to DNA and has been shown to cause mutation.[1] It is also used in combination with isocytosine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.[2][3]
It is used as a nucleobase of hachimoji nucleic acids.[4] In hachimoji DNA, it pairs with 1-methylcytosine, while in hachimoji RNA, it pairs with isocytosine.

References
- ^ Yang XL, Sugiyama H, Ikeda S, Saito I, Wang AH (1998). "Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy". Biophys. J. 75 (3): 1163–1171. doi:10.1016/S0006-3495(98)74035-4. PMC 1299791. PMID 9726918.
- ^ Andrzej Jaworski, Józef S. Kwiatkowski, Bogdan Lesyng: „Why isoguanine and isocytosine are not the components of the genetic code", International Journal of Quantum Chemistry, Supplement: Proceedings of the International Symposium on Quantum Biology and Quantum Pharmacology, 1985, 28 (Supplement S12), pp. 209–216 (doi:10.1002/qua.560280720).
- ^ Christopher Roberts, Rajanikanth Bandaru, Christopher Switzer: „Theoretical and Experimental Study of Isoguanine and Isocytosine: Base Pairing in an Expanded Genetic System", J. Am. Chem. Soc., 1997, 119 (20), pp. 4640–4649 (doi:10.1021/ja970123s).
- ^ Hoshika, Shuichi; et al. (22 February 2019). "Hachimoji DNA and RNA: A genetic system with eight building blocks". Science. 363 (6429): 884–887. doi:10.1126/science.aat0971.
{{cite journal}}: Unknown parameter|subscription=ignored (|url-access=suggested) (help)
