K-mer: Difference between revisions
mNo edit summary |
Add section on forces affecting DNA k-mer frequencies |
||
| Line 2: | Line 2: | ||
{{DISPLAYTITLE:''k''-mer}} |
{{DISPLAYTITLE:''k''-mer}} |
||
[[File:K-mer diagram.svg|thumb|The sequence ATGG has two 3-mers: ATG and TGG.]] |
[[File:K-mer diagram.svg|thumb|The sequence ATGG has two 3-mers: ATG and TGG.]] |
||
In [[bioinformatics]], '''''k''-mers''' are [[substring|subsequence]]s of length <math>k</math> contained within a biological sequence. Primarily used within the context of [[computational genomics]] and [[sequence analysis]], in which ''k''-mers are composed of [[Nucleotide|nucleotides]] (''i.e''. A, T, G, and C), ''k''-mers are capitalized upon to [[Sequence assembly|assemble DNA sequences]],<ref>{{Cite journal|last=Compeau|first=Phillip E C|last2=Pevzner|first2=Pavel A|last3=Tesler|first3=Glenn|date=2011-11|title=How to apply de Bruijn graphs to genome assembly|url=http://www.nature.com/articles/nbt.2023|journal=Nature Biotechnology|language=en|volume=29|issue=11|pages=987–991|doi=10.1038/nbt.2023|issn=1087-0156}}</ref> improve [[Protein production|heterologous gene expression]],<ref>{{Cite journal|last=Welch|first=Mark|last2=Govindarajan|first2=Sridhar|last3=Ness|first3=Jon E.|last4=Villalobos|first4=Alan|last5=Gurney|first5=Austin|last6=Minshull|first6=Jeremy|last7=Gustafsson|first7=Claes|date=2009-09-14|editor-last=Kudla|editor-first=Grzegorz|title=Design Parameters to Control Synthetic Gene Expression in Escherichia coli|url=https://dx.plos.org/10.1371/journal.pone.0007002|journal=PLoS ONE|language=en|volume=4|issue=9|pages=e7002|doi=10.1371/journal.pone.0007002|issn=1932-6203}}</ref><ref>{{Cite journal|last=Gustafsson|first=Claes|last2=Govindarajan|first2=Sridhar|last3=Minshull|first3=Jeremy|date=2004-7|title=Codon bias and heterologous protein expression|url=https://linkinghub.elsevier.com/retrieve/pii/S0167779904001118|journal=Trends in Biotechnology|language=en|volume=22|issue=7|pages=346–353|doi=10.1016/j.tibtech.2004.04.006}}</ref> [[Binning (metagenomics)|identify species in metagenomic samples]],<ref>{{Cite journal|last=Perry|first=Scott C.|last2=Beiko|first2=Robert G.|date=2010-01-01|title=Distinguishing Microbial Genome Fragments Based on Their Composition: Evolutionary and Comparative Genomic Perspectives|url=https://academic.oup.com/gbe/article/doi/10.1093/gbe/evq004/568285|journal=Genome Biology and Evolution|language=en|volume=2|pages=117–131|doi=10.1093/gbe/evq004|issn=1759-6653}}</ref> and create [[Attenuated vaccine|attenuated vaccines]].<ref>{{Cite journal|last=Eschke|first=Kathrin|last2=Trimpert|first2=Jakob|last3=Osterrieder|first3=Nikolaus|last4=Kunec|first4=Dusan|date=2018-01-29|editor-last=Mocarski|editor-first=Edward|title=Attenuation of a very virulent Marek's disease herpesvirus (MDV) by codon pair bias deoptimization|url=https://dx.plos.org/10.1371/journal.ppat.1006857|journal=PLOS Pathogens|language=en|volume=14|issue=1|pages=e1006857|doi=10.1371/journal.ppat.1006857|issn=1553-7374}}</ref> Usually, the term ''k''-mer refers to all of a sequence's subsequences of length <math>k</math>, such that the sequence AGAT would have four [[Monomer|monomers]] (A, G, A, and T), three 2-mers (AG, GA, AT), two 3-mers (AGA and GAT) and one 4-mer (AGAT). More generally, a sequence of length <math>L</math> will have <math>L - k + 1</math> ''k''-mers and <math>n^{k}</math> total possible ''k''-mers, where <math>n</math> is number of possible monomers (e.g. four in the case of [[DNA]]). |
In [[bioinformatics]], '''''k''-mers''' are [[substring|subsequence]]s of length <math>k</math> contained within a biological sequence. Primarily used within the context of [[computational genomics]] and [[sequence analysis]], in which ''k''-mers are composed of [[Nucleotide|nucleotides]] (''i.e''. A, T, G, and C), ''k''-mers are capitalized upon to [[Sequence assembly|assemble DNA sequences]],<ref>{{Cite journal|last=Compeau|first=Phillip E C|last2=Pevzner|first2=Pavel A|last3=Tesler|first3=Glenn|date=2011-11|title=How to apply de Bruijn graphs to genome assembly|url=http://www.nature.com/articles/nbt.2023|journal=Nature Biotechnology|language=en|volume=29|issue=11|pages=987–991|doi=10.1038/nbt.2023|issn=1087-0156}}</ref> improve [[Protein production|heterologous gene expression]],<ref>{{Cite journal|last=Welch|first=Mark|last2=Govindarajan|first2=Sridhar|last3=Ness|first3=Jon E.|last4=Villalobos|first4=Alan|last5=Gurney|first5=Austin|last6=Minshull|first6=Jeremy|last7=Gustafsson|first7=Claes|date=2009-09-14|editor-last=Kudla|editor-first=Grzegorz|title=Design Parameters to Control Synthetic Gene Expression in Escherichia coli|url=https://dx.plos.org/10.1371/journal.pone.0007002|journal=PLoS ONE|language=en|volume=4|issue=9|pages=e7002|doi=10.1371/journal.pone.0007002|issn=1932-6203}}</ref><ref>{{Cite journal|last=Gustafsson|first=Claes|last2=Govindarajan|first2=Sridhar|last3=Minshull|first3=Jeremy|date=2004-7|title=Codon bias and heterologous protein expression|url=https://linkinghub.elsevier.com/retrieve/pii/S0167779904001118|journal=Trends in Biotechnology|language=en|volume=22|issue=7|pages=346–353|doi=10.1016/j.tibtech.2004.04.006}}</ref> [[Binning (metagenomics)|identify species in metagenomic samples]],<ref name=":0">{{Cite journal|last=Perry|first=Scott C.|last2=Beiko|first2=Robert G.|date=2010-01-01|title=Distinguishing Microbial Genome Fragments Based on Their Composition: Evolutionary and Comparative Genomic Perspectives|url=https://academic.oup.com/gbe/article/doi/10.1093/gbe/evq004/568285|journal=Genome Biology and Evolution|language=en|volume=2|pages=117–131|doi=10.1093/gbe/evq004|issn=1759-6653}}</ref> and create [[Attenuated vaccine|attenuated vaccines]].<ref>{{Cite journal|last=Eschke|first=Kathrin|last2=Trimpert|first2=Jakob|last3=Osterrieder|first3=Nikolaus|last4=Kunec|first4=Dusan|date=2018-01-29|editor-last=Mocarski|editor-first=Edward|title=Attenuation of a very virulent Marek's disease herpesvirus (MDV) by codon pair bias deoptimization|url=https://dx.plos.org/10.1371/journal.ppat.1006857|journal=PLOS Pathogens|language=en|volume=14|issue=1|pages=e1006857|doi=10.1371/journal.ppat.1006857|issn=1553-7374}}</ref> Usually, the term ''k''-mer refers to all of a sequence's subsequences of length <math>k</math>, such that the sequence AGAT would have four [[Monomer|monomers]] (A, G, A, and T), three 2-mers (AG, GA, AT), two 3-mers (AGA and GAT) and one 4-mer (AGAT). More generally, a sequence of length <math>L</math> will have <math>L - k + 1</math> ''k''-mers and <math>n^{k}</math> total possible ''k''-mers, where <math>n</math> is number of possible monomers (e.g. four in the case of [[DNA]]). |
||
== Introduction == |
== Introduction == |
||
| Line 45: | Line 45: | ||
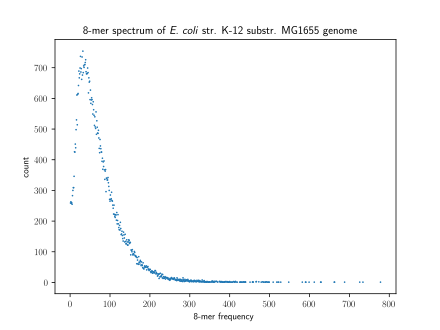
A method of visualizing ''k''-mers, the '''''k''-mer spectrum''', shows the multiplicity of each ''k''-mer in a sequence versus the number of ''k''-mers with that multiplicity.<ref>{{Cite journal|last=Mapleson|first=Daniel|last2=Garcia Accinelli|first2=Gonzalo|last3=Kettleborough|first3=George|last4=Wright|first4=Jonathan|last5=Clavijo|first5=Bernardo J.|date=2016-10-22|title=KAT: a K-mer analysis toolkit to quality control NGS datasets and genome assemblies|url=https://academic.oup.com/bioinformatics/article-lookup/doi/10.1093/bioinformatics/btw663|journal=Bioinformatics|language=en|pages=btw663|doi=10.1093/bioinformatics/btw663|issn=1367-4803}}</ref> The number of modes in a ''k''-mer spectrum for a species's genome varies, with most species having a unimodal distribution.<ref>{{Cite journal|last=Chor|first=Benny|last2=Horn|first2=David|last3=Goldman|first3=Nick|last4=Levy|first4=Yaron|last5=Massingham|first5=Tim|date=2009|title=Genomic DNA k-mer spectra: models and modalities|url=http://genomebiology.biomedcentral.com/articles/10.1186/gb-2009-10-10-r108|journal=Genome Biology|language=en|volume=10|issue=10|pages=R108|doi=10.1186/gb-2009-10-10-r108|issn=1465-6906}}</ref> However, all [[Mammal|mammals]] have a multimodal distribution. The number of modes within a ''k''-mer spectrum can vary between regions of genomes as well: humans have unimodal ''k''-mer spectra in [[Five prime untranslated region|5' UTRs]] and [[Exon|exons]] but multimodal spectra in [[Three prime untranslated region|3' UTRs]] and [[Intron|introns]]. |
A method of visualizing ''k''-mers, the '''''k''-mer spectrum''', shows the multiplicity of each ''k''-mer in a sequence versus the number of ''k''-mers with that multiplicity.<ref>{{Cite journal|last=Mapleson|first=Daniel|last2=Garcia Accinelli|first2=Gonzalo|last3=Kettleborough|first3=George|last4=Wright|first4=Jonathan|last5=Clavijo|first5=Bernardo J.|date=2016-10-22|title=KAT: a K-mer analysis toolkit to quality control NGS datasets and genome assemblies|url=https://academic.oup.com/bioinformatics/article-lookup/doi/10.1093/bioinformatics/btw663|journal=Bioinformatics|language=en|pages=btw663|doi=10.1093/bioinformatics/btw663|issn=1367-4803}}</ref> The number of modes in a ''k''-mer spectrum for a species's genome varies, with most species having a unimodal distribution.<ref>{{Cite journal|last=Chor|first=Benny|last2=Horn|first2=David|last3=Goldman|first3=Nick|last4=Levy|first4=Yaron|last5=Massingham|first5=Tim|date=2009|title=Genomic DNA k-mer spectra: models and modalities|url=http://genomebiology.biomedcentral.com/articles/10.1186/gb-2009-10-10-r108|journal=Genome Biology|language=en|volume=10|issue=10|pages=R108|doi=10.1186/gb-2009-10-10-r108|issn=1465-6906}}</ref> However, all [[Mammal|mammals]] have a multimodal distribution. The number of modes within a ''k''-mer spectrum can vary between regions of genomes as well: humans have unimodal ''k''-mer spectra in [[Five prime untranslated region|5' UTRs]] and [[Exon|exons]] but multimodal spectra in [[Three prime untranslated region|3' UTRs]] and [[Intron|introns]]. |
||
== Forces Affecting DNA ''k''-mer Frequency == |
|||
The frequency of ''k''-mer usage is affected by numerous forces, working at multiple levels, which are often in conflict. It is important to note that ''k''-mers for higher values of ''k'' are affected by the forces affecting lower values of ''k'' as well. For example, if the 1-mer A does not occur in a sequence, none of the 2-mers containing A (AA, AT, AG, and AC) will occur either, thereby linking the effects of the different forces. |
|||
=== ''k'' = 1 === |
|||
When ''k'' = 1, there are four DNA ''k''-mers, ''i.e.'', A, T, G, and C. At the molecular level, there are three [[Hydrogen bond|hydrogen bonds]] between G and C, whereas there are only two between A and T. GC bonds, as a result of the extra hydrogen bond (and stronger stacking interactions), are more thermally stable than AT bonds.<ref>{{Cite journal|last=Yakovchuk|first=P.|date=2006-01-30|title=Base-stacking and base-pairing contributions into thermal stability of the DNA double helix|url=https://academic.oup.com/nar/article-lookup/doi/10.1093/nar/gkj454|journal=Nucleic Acids Research|language=en|volume=34|issue=2|pages=564–574|doi=10.1093/nar/gkj454|issn=0305-1048}}</ref> Mammals and birds have a higher ratio of Gs and Cs to As and Ts ([[GC-content]]), which led to the hypothesis that thermal stability was a driving factor of GC-content variation.<ref>{{Cite journal|last=Bernardi|first=Giorgio|date=2000-1|title=Isochores and the evolutionary genomics of vertebrates|url=https://linkinghub.elsevier.com/retrieve/pii/S0378111999004850|journal=Gene|language=en|volume=241|issue=1|pages=3–17|doi=10.1016/S0378-1119(99)00485-0}}</ref> However, while promising, this hypothesis did not hold up under scrutiny: analysis among a variety of prokaryotes showed no evidence of GC-content correlating with temperature as the thermal adaptation hypothesis would predict.<ref>{{Cite journal|last=Hurst|first=Laurence D.|last2=Merchant|first2=Alexa R.|date=2001-03-07|title=High guanine–cytosine content is not an adaptation to high temperature: a comparative analysis amongst prokaryotes|url=http://www.royalsocietypublishing.org/doi/10.1098/rspb.2000.1397|journal=Proceedings of the Royal Society of London. Series B: Biological Sciences|language=en|volume=268|issue=1466|pages=493–497|doi=10.1098/rspb.2000.1397|issn=1471-2954}}</ref> Indeed, if natural selection were to be the driving force behind GC-content variation, that would require that [[Single nucleotide polymorphism|single nucleotide changes]], which are often [[Synonymous substitution|silent]], to alter the fitness of an organism.<ref>{{Cite journal|last=Mugal|first=Carina F.|last2=Weber|first2=Claudia C.|last3=Ellegren|first3=Hans|date=2015-12|title=GC-biased gene conversion links the recombination landscape and demography to genomic base composition: GC-biased gene conversion drives genomic base composition across a wide range of species|url=http://doi.wiley.com/10.1002/bies.201500058|journal=BioEssays|language=en|volume=37|issue=12|pages=1317–1326|doi=10.1002/bies.201500058}}</ref> |
|||
Rather, current evidence suggests that [[Gene conversion#GC-biased gene conversion|GC‐biased gene conversion]] (gBGC) is a driving factor behind variation in GC content.<ref>{{Cite journal|last=Mugal|first=Carina F.|last2=Weber|first2=Claudia C.|last3=Ellegren|first3=Hans|date=2015-12|title=GC-biased gene conversion links the recombination landscape and demography to genomic base composition: GC-biased gene conversion drives genomic base composition across a wide range of species|url=http://doi.wiley.com/10.1002/bies.201500058|journal=BioEssays|language=en|volume=37|issue=12|pages=1317–1326|doi=10.1002/bies.201500058}}</ref> gBGC is a process that occurs during [[Genetic recombination|recombination]] which replaces Gs and Cs with As and Ts.<ref>{{Cite journal|last=Romiguier|first=Jonathan|last2=Roux|first2=Camille|date=2017-02-15|title=Analytical Biases Associated with GC-Content in Molecular Evolution|url=http://journal.frontiersin.org/article/10.3389/fgene.2017.00016/full|journal=Frontiers in Genetics|volume=8|doi=10.3389/fgene.2017.00016|issn=1664-8021}}</ref> This process, though distinct from natural selection, can nevertheless exert selective pressure on DNA biased towards GC replacements being fixed in the genome. gBGC can therefore be seen as an "impostor" of natural selection. As would be expected, GC content is greater at sites experiencing greater recombination.<ref>{{Cite journal|last=Spencer|first=C.C.A.|date=2006-08-01|title=Human polymorphism around recombination hotspots: Figure 1|url=http://www.biochemsoctrans.org/cgi/doi/10.1042/BST0340535|journal=Biochemical Society Transactions|language=en|volume=34|issue=4|pages=535–536|doi=10.1042/BST0340535|issn=0300-5127}}</ref> Furthermore, organisms with higher rates of recombination exhibit higher GC content, in keeping with the gBGC hypothesis's predicted effects.<ref>{{Cite journal|last=Weber|first=Claudia C|last2=Boussau|first2=Bastien|last3=Romiguier|first3=Jonathan|last4=Jarvis|first4=Erich D|last5=Ellegren|first5=Hans|date=2014-12|title=Evidence for GC-biased gene conversion as a driver of between-lineage differences in avian base composition|url=http://genomebiology.biomedcentral.com/articles/10.1186/s13059-014-0549-1|journal=Genome Biology|language=en|volume=15|issue=12|doi=10.1186/s13059-014-0549-1|issn=1474-760X}}</ref> Interestingly, gBGC does not appear to be limited to [[eukaryotes]].<ref>{{Cite journal|last=Lassalle|first=Florent|last2=Périan|first2=Séverine|last3=Bataillon|first3=Thomas|last4=Nesme|first4=Xavier|last5=Duret|first5=Laurent|last6=Daubin|first6=Vincent|date=2015-02-06|editor-last=Petrov|editor-first=Dmitri A.|title=GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands|url=https://dx.plos.org/10.1371/journal.pgen.1004941|journal=PLOS Genetics|language=en|volume=11|issue=2|pages=e1004941|doi=10.1371/journal.pgen.1004941|issn=1553-7404}}</ref> Asexual organisms such as bacteria and archaea also experience recombination by means of gene conversion, a process of homologous sequence replacement resulting in multiple identical sequences throughout the genome.<ref>{{Cite journal|last=Santoyo|first=G|last2=Romero|first2=D|date=2005-4|title=Gene conversion and concerted evolution in bacterial genomes|url=http://doi.wiley.com/10.1016/j.femsre.2004.10.004|journal=FEMS Microbiology Reviews|language=en|volume=29|issue=2|pages=169–183|doi=10.1016/j.femsre.2004.10.004}}</ref> That recombination is able to drive up GC content in all domains of life suggests that gBGC is universally conserved. Whether gBGC is a (mostly) neutral byproduct of the molecular machinery of life or is itself under selection remains to be determined. The exact mechanism and evolutionary advantage or disadvantage of gBGC is currently unknown.<ref>{{Citation|last=Bhérer|first=Claude|title=Biased Gene Conversion and Its Impact on Genome Evolution|date=2014-06-16|url=http://doi.wiley.com/10.1002/9780470015902.a0020834.pub2|work=eLS|editor-last=John Wiley & Sons Ltd|publisher=John Wiley & Sons, Ltd|language=en|doi=10.1002/9780470015902.a0020834.pub2|isbn=9780470015902|access-date=2019-06-23|last2=Auton|first2=Adam}}</ref> |
|||
=== ''k'' = 2 === |
|||
Despite the comparatively large body of literature discussing GC-content biases, relatively little has been written about dinucleotide biases. What is known is that these dinucleotide biases are relatively constant throughout the genome, unlike GC-content, which, as seen above, can vary considerably.<ref>{{Cite journal|last=Karlin|first=Samuel|date=1998-10|title=Global dinucleotide signatures and analysis of genomic heterogeneity|url=https://linkinghub.elsevier.com/retrieve/pii/S1369527498800957|journal=Current Opinion in Microbiology|language=en|volume=1|issue=5|pages=598–610|doi=10.1016/S1369-5274(98)80095-7}}</ref> This is an important insight that must not be overlooked. If dinucleotide bias were subject to pressures resulting from [[Translation (biology)|translation]], then we would expect to see differing patterns of dinucleotide bias in [[Coding region|coding]] and [[Non-coding DNA|noncoding]] regions driven by some dinucelotides' reduced translational efficiency.<ref>{{Cite journal|last=Beutler|first=E.|last2=Gelbart|first2=T.|last3=Han|first3=J. H.|last4=Koziol|first4=J. A.|last5=Beutler|first5=B.|date=1989-01-01|title=Evolution of the genome and the genetic code: selection at the dinucleotide level by methylation and polyribonucleotide cleavage.|url=http://www.pnas.org/cgi/doi/10.1073/pnas.86.1.192|journal=Proceedings of the National Academy of Sciences|language=en|volume=86|issue=1|pages=192–196|doi=10.1073/pnas.86.1.192|issn=0027-8424}}</ref> Because we do not, we can therefore infer that the forces modulating dinucleotide bias are independent of translation. Further evidence against translational pressures affecting dinucleotide bias is the fact that the dinucleotide biases of viruses, which rely heavily on translational efficiency, are shaped by their viral family more than by their hosts, whose translational machinery the viruses hijack.<ref>{{Cite journal|last=Di Giallonardo|first=Francesca|last2=Schlub|first2=Timothy E.|last3=Shi|first3=Mang|last4=Holmes|first4=Edward C.|date=2017-04-15|editor-last=Dermody|editor-first=Terence S.|title=Dinucleotide Composition in Animal RNA Viruses Is Shaped More by Virus Family than by Host Species|url=http://jvi.asm.org/lookup/doi/10.1128/JVI.02381-16|journal=Journal of Virology|language=en|volume=91|issue=8|doi=10.1128/JVI.02381-16|issn=0022-538X}}</ref> |
|||
Counter to gBGC's increasing GC-content is [[CG suppression]], which reduces the frequency of [[CpG site|CG]] 2-mers due to [[deamination]] of [[Methylation#DNA/RNA methylation|methylated]] CG dinucleotides, resulting in substitutions of CGs with TGs, thereby reducing the GC-content.<ref>{{Cite journal|last=Żemojtel|first=Tomasz|last2=kiełbasa|first2=Szymon M.|last3=Arndt|first3=Peter F.|last4=Behrens|first4=Sarah|last5=Bourque|first5=Guillaume|last6=Vingron|first6=Martin|date=2011-01-01|title=CpG Deamination Creates Transcription Factor–Binding Sites with High Efficiency|url=https://academic.oup.com/gbe/article/doi/10.1093/gbe/evr107/596611|journal=Genome Biology and Evolution|language=en|volume=3|pages=1304–1311|doi=10.1093/gbe/evr107|issn=1759-6653}}</ref> This interaction highlights the interrelationship between the forces affecting ''k''-mers for varying values of ''k.'' |
|||
One interesting fact about dinucleotide bias is that it can serve as a "distance" measurement between phylogenetically similar genomes. The genomes of pairs of organisms that are closely related share more similar dinucleotide biases than between pairs of more distantly related organisms.<ref>{{Cite journal|last=Karlin|first=Samuel|date=1998-10|title=Global dinucleotide signatures and analysis of genomic heterogeneity|url=https://linkinghub.elsevier.com/retrieve/pii/S1369527498800957|journal=Current Opinion in Microbiology|language=en|volume=1|issue=5|pages=598–610|doi=10.1016/S1369-5274(98)80095-7}}</ref> |
|||
=== ''k'' = 3 === |
|||
There are twenty natural [[Amino acid|amino acids]] that are used to build the proteins that DNA encodes. However, there are only four nucleotides. Therefore, there cannot be a one-to-one correspondence between nucleotides and amino acids. Similarly, there are 16 2-mers, which is also not enough to unambiguously represent every amino acid. However, there are 64 distinct 3-mers in DNA, which is enough to uniquely represent each amino acid. These non-overlapping 3-mers are called [[Genetic code|codons]]. While each codon only maps to one amino acid, each amino acid can be [[Codon degeneracy|represented by multiple codons]]. Thus, the same amino acid sequence can have multiple DNA representations. Interestingly, each codon for an amino acid is not used in equal proportions.<ref name=":2">Hershberg R, Petrov DA. Selection on Codon Bias. Annual Review of Genetics. Annual Reviews; 2008;42: 287–299. doi:10.1146/annurev.genet.42.110807.091442</ref> This is called [[Codon usage bias|codon-usage bias]] (CUB). As a parenthetical, when ''k'' = 3 we must distinguish between true 3-mer frequency and CUB. For example, the sequence ATGGCA has four ''k''-mer words within it (ATG, TGG, GGC, and GCA) while only containing two codons (ATG and GCA). However, CUB is a major driving factor of 3-mer usage bias (accounting for up to ⅓ of it, since ⅓ of the ''k''-mers in a coding region are codons) and will be the main focus of this section. |
|||
The exact cause of variation between the frequencies of various codons is not fully understood. It is known that codon preference is correlated with tRNA abundances, with codons matching more abundant tRNAs being correspondingly more frequent<ref name=":2" /> and that more highly expressed proteins exhibit greater CUB.<ref>{{Cite journal|last=Sharp|first=Paul M.|last2=Li|first2=Wen-Hsiung|date=1987|title=The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications|url=https://academic.oup.com/nar/article-lookup/doi/10.1093/nar/15.3.1281|journal=Nucleic Acids Research|language=en|volume=15|issue=3|pages=1281–1295|doi=10.1093/nar/15.3.1281|issn=0305-1048}}</ref> This suggests that selection for translational efficiency or accuracy is the driving force behind CUB variation. |
|||
=== ''k'' = 4 === |
|||
Similar to the effect seen in dinucleotide bias, the tetranucleotide biases of phylogenetically similar organisms are more similar than between less closely related organisms.<ref name=":0" /> The exact cause of variation in tetranucleotide bias is not well understood, but it has been hypothesized to be the result of the maintenance of genetic stability at the molecular level.<ref>{{Cite journal|last=Noble|first=Peter A.|last2=Citek|first2=Robert W.|last3=Ogunseitan|first3=Oladele A.|date=1998-04|title=Tetranucleotide frequencies in microbial genomes|url=http://dx.doi.org/10.1002/elps.1150190412|journal=Electrophoresis|volume=19|issue=4|pages=528–535|doi=10.1002/elps.1150190412|issn=0173-0835}}</ref> |
|||
== Sequence assembly== |
== Sequence assembly== |
||
Revision as of 17:39, 23 June 2019

In bioinformatics, k-mers are subsequences of length contained within a biological sequence. Primarily used within the context of computational genomics and sequence analysis, in which k-mers are composed of nucleotides (i.e. A, T, G, and C), k-mers are capitalized upon to assemble DNA sequences,[1] improve heterologous gene expression,[2][3] identify species in metagenomic samples,[4] and create attenuated vaccines.[5] Usually, the term k-mer refers to all of a sequence's subsequences of length , such that the sequence AGAT would have four monomers (A, G, A, and T), three 2-mers (AG, GA, AT), two 3-mers (AGA and GAT) and one 4-mer (AGAT). More generally, a sequence of length will have k-mers and total possible k-mers, where is number of possible monomers (e.g. four in the case of DNA).
Introduction
k-mers are simply length subsequences. For example, all the possible k-mers of a DNA sequence are shown below:
| k | k-mers |
|---|---|
| 1 | G, T, A, G, A, G, C, T, G, T |
| 2 | GT, TA, AG, GA, AG, GC, CT, TG, GT |
| 3 | GTA, TAG, AGA, GAG, AGC, GCT, CTG, TGT |
| 4 | GTAG, TAGA, AGAG, GAGC, AGCT, GCTG, CTGT |
| 5 | GTAGA, TAGAG, AGAGC, GAGCT, AGCTG, GCTGT |
| 6 | GTAGAG, TAGAGC, AGAGCT, GAGCTG, AGCTGT |
| 7 | GTAGAGC, TAGAGCT, AGAGCTG, GAGCTGT |
| 8 | GTAGAGCT, TAGAGCTG, AGAGCTGT |
| 9 | GTAGAGCTG, TAGAGCTGT |
| 10 | GTAGAGCTGT |
A method of visualizing k-mers, the k-mer spectrum, shows the multiplicity of each k-mer in a sequence versus the number of k-mers with that multiplicity.[6] The number of modes in a k-mer spectrum for a species's genome varies, with most species having a unimodal distribution.[7] However, all mammals have a multimodal distribution. The number of modes within a k-mer spectrum can vary between regions of genomes as well: humans have unimodal k-mer spectra in 5' UTRs and exons but multimodal spectra in 3' UTRs and introns.
Forces Affecting DNA k-mer Frequency
The frequency of k-mer usage is affected by numerous forces, working at multiple levels, which are often in conflict. It is important to note that k-mers for higher values of k are affected by the forces affecting lower values of k as well. For example, if the 1-mer A does not occur in a sequence, none of the 2-mers containing A (AA, AT, AG, and AC) will occur either, thereby linking the effects of the different forces.
k = 1
When k = 1, there are four DNA k-mers, i.e., A, T, G, and C. At the molecular level, there are three hydrogen bonds between G and C, whereas there are only two between A and T. GC bonds, as a result of the extra hydrogen bond (and stronger stacking interactions), are more thermally stable than AT bonds.[8] Mammals and birds have a higher ratio of Gs and Cs to As and Ts (GC-content), which led to the hypothesis that thermal stability was a driving factor of GC-content variation.[9] However, while promising, this hypothesis did not hold up under scrutiny: analysis among a variety of prokaryotes showed no evidence of GC-content correlating with temperature as the thermal adaptation hypothesis would predict.[10] Indeed, if natural selection were to be the driving force behind GC-content variation, that would require that single nucleotide changes, which are often silent, to alter the fitness of an organism.[11]
Rather, current evidence suggests that GC‐biased gene conversion (gBGC) is a driving factor behind variation in GC content.[12] gBGC is a process that occurs during recombination which replaces Gs and Cs with As and Ts.[13] This process, though distinct from natural selection, can nevertheless exert selective pressure on DNA biased towards GC replacements being fixed in the genome. gBGC can therefore be seen as an "impostor" of natural selection. As would be expected, GC content is greater at sites experiencing greater recombination.[14] Furthermore, organisms with higher rates of recombination exhibit higher GC content, in keeping with the gBGC hypothesis's predicted effects.[15] Interestingly, gBGC does not appear to be limited to eukaryotes.[16] Asexual organisms such as bacteria and archaea also experience recombination by means of gene conversion, a process of homologous sequence replacement resulting in multiple identical sequences throughout the genome.[17] That recombination is able to drive up GC content in all domains of life suggests that gBGC is universally conserved. Whether gBGC is a (mostly) neutral byproduct of the molecular machinery of life or is itself under selection remains to be determined. The exact mechanism and evolutionary advantage or disadvantage of gBGC is currently unknown.[18]
k = 2
Despite the comparatively large body of literature discussing GC-content biases, relatively little has been written about dinucleotide biases. What is known is that these dinucleotide biases are relatively constant throughout the genome, unlike GC-content, which, as seen above, can vary considerably.[19] This is an important insight that must not be overlooked. If dinucleotide bias were subject to pressures resulting from translation, then we would expect to see differing patterns of dinucleotide bias in coding and noncoding regions driven by some dinucelotides' reduced translational efficiency.[20] Because we do not, we can therefore infer that the forces modulating dinucleotide bias are independent of translation. Further evidence against translational pressures affecting dinucleotide bias is the fact that the dinucleotide biases of viruses, which rely heavily on translational efficiency, are shaped by their viral family more than by their hosts, whose translational machinery the viruses hijack.[21]
Counter to gBGC's increasing GC-content is CG suppression, which reduces the frequency of CG 2-mers due to deamination of methylated CG dinucleotides, resulting in substitutions of CGs with TGs, thereby reducing the GC-content.[22] This interaction highlights the interrelationship between the forces affecting k-mers for varying values of k.
One interesting fact about dinucleotide bias is that it can serve as a "distance" measurement between phylogenetically similar genomes. The genomes of pairs of organisms that are closely related share more similar dinucleotide biases than between pairs of more distantly related organisms.[23]
k = 3
There are twenty natural amino acids that are used to build the proteins that DNA encodes. However, there are only four nucleotides. Therefore, there cannot be a one-to-one correspondence between nucleotides and amino acids. Similarly, there are 16 2-mers, which is also not enough to unambiguously represent every amino acid. However, there are 64 distinct 3-mers in DNA, which is enough to uniquely represent each amino acid. These non-overlapping 3-mers are called codons. While each codon only maps to one amino acid, each amino acid can be represented by multiple codons. Thus, the same amino acid sequence can have multiple DNA representations. Interestingly, each codon for an amino acid is not used in equal proportions.[24] This is called codon-usage bias (CUB). As a parenthetical, when k = 3 we must distinguish between true 3-mer frequency and CUB. For example, the sequence ATGGCA has four k-mer words within it (ATG, TGG, GGC, and GCA) while only containing two codons (ATG and GCA). However, CUB is a major driving factor of 3-mer usage bias (accounting for up to ⅓ of it, since ⅓ of the k-mers in a coding region are codons) and will be the main focus of this section.
The exact cause of variation between the frequencies of various codons is not fully understood. It is known that codon preference is correlated with tRNA abundances, with codons matching more abundant tRNAs being correspondingly more frequent[24] and that more highly expressed proteins exhibit greater CUB.[25] This suggests that selection for translational efficiency or accuracy is the driving force behind CUB variation.
k = 4
Similar to the effect seen in dinucleotide bias, the tetranucleotide biases of phylogenetically similar organisms are more similar than between less closely related organisms.[4] The exact cause of variation in tetranucleotide bias is not well understood, but it has been hypothesized to be the result of the maintenance of genetic stability at the molecular level.[26]
Sequence assembly
Overview
In sequence assembly, k-mers are typically used during the construction of De Bruijn graphs. In order to create a De Bruijn Graph, the strings stored in each edge with length, , must overlap another string in another edge by in order to create a vertex. Reads generated from next-generation sequencing will typically have different read lengths being generated. For example, reads by Illumina’s sequencing technology capture reads of 100-mers. However, the problem with the sequencing is that only small fractions out of all the possible 100-mers that are present in the genome are actually generated. This is due to read errors, but more importantly, just simple coverage holes that occur during sequencing. The problem is that these small fractions of the possible k-mers violate the key assumption of de Bruijn graphs that all the k-mer reads must overlap its adjoining k-mer in the genome by (which can’t occur when all the possible k-mers aren’t present). The solution to this problem is to break these k-mer sized reads into smaller k-mers, such that the resulting smaller k-mers will represent all the possible k-mers of that smaller size that are present in the genome.[27] Furthermore, splitting the k-mers into smaller sizes also helps alleviate the problem of different initial read lengths. An example of the solution of splitting the reads into smaller k-mers is shown in figure 1. In this example the 5 reads do not account for all the possible 7-mers of the genome, and as such, a de Bruijn graph cannot be created. But when they are split into 4-mers, the resultant subsequences are enough to reconstruct the genome using a de Bruijn graph.

Choice of k-mer
The choice of the k-mer size has many different effects on the sequence assembly. These effects vary greatly between lower sized and larger sized k-mers. Therefore, an understanding of the different k-mer sizes must be achieved in order to choose a suitable size that balances the effects. The effects of the sizes are outlined below.
Lower k-mer sizes
- A lower k-mer size will decrease the amount of edges stored in the graph, and as such, will help decrease the amount of space required to store DNA sequence.
- Having smaller sizes will increase the chance for all the k-mers to overlap, and as such, have the required subsequences in order to construct the de Bruijn graph.[28]
- However, by having smaller sized k-mers, you also risk having many vertices in the graph leading into a single k-mer. Therefore, this will make the reconstruction of the genome more difficult as there is a higher level of path ambiguities due to the larger amount of vertices that will need to be traversed.
- Information is lost as the k-mers become smaller.
- E.g. The possibility of AGTCGTAGATGCTG is lower than ACGT, and as such, holds a greater amount of information (refer to entropy (information theory) for more information).
- Smaller k-mers also have the problem of not being able to resolve areas in the DNA where small microsatellites or repeats occur. This is because smaller k-mers will tend to sit entirely within the repeat region and is therefore hard to determine the amount of repetition that has actually taken place.
- E.g. For the subsequence ATGTGTGTGTGTGTACG, the amount of repetitions of TG will be lost if a k-mer size less than 16 is chosen. This is because most of the k-mers will sit in the repeated region and may just be discarded as repeats of the same k-mer instead of referring the amount of repeats.
Higher k-mer sizes
- Having larger sized k-mers will increase the amount of edges in the graph, which in turn, will increase the amount of memory needed to store the DNA sequence.
- By increasing the size of the k-mers, the number of vertices will also decrease. This will help with the construction of the genome as there will be fewer paths to traverse in the graph.[28]
- Larger k-mers also run a higher risk of not having outward vertices from every k-mer. This is due to larger k-mers increasing the risk that it will not overlap with another k-mer by . Therefore, this can lead to disjoints in the reads, and as such, can lead to a higher amount of smaller contigs.
- Larger k-mer sizes help alleviate the problem of small repeat regions. This is due to the fact that the k-mer will contain a balance of the repeat region and the adjoining DNA sequences (given it are a large enough size) that can help to resolve the amount of repetition in that particular area.
Applications of k-mer in bioinformatics analysis
The frequency of a set of k-mers in a species' genome, in a genomic region, or in a class of sequences, can be used as a "signature" of the underlying sequence. Comparing these frequencies are computationally easier than sequence alignment, and is an important method in alignment-free sequence analysis. It can also be used as a first stage analysis before an alignment.
- separating different species in a mixture of genetic material (metagenomics, microbiome);[29][30] phase/frame information can be added[31]
- DNA barcoding of species[32][33]
- de novo sequence assembly[34]
- human mitochondrial haplogroup classification [35]
- detect genome mis-assembly[36]
- de novo detection of repeated sequence such as transposable element[37]
- characterize a protein-binding sequence motif.[38] Besides k-mer, gapped k-mers (also named gapped q-grams[39] or spaced seeds[40]) can also be used [41]
- identification of mutation or polymorphism using next generation sequencing data[42]
- characterizing CpG island by the flanking regions [43][44]
- detect horizontal transfer [45]
- detect bacterial contamination in eukaryotic genome assembly [46][47]
- detect recombination site [48]
- using the k-mer frequency vs k-mer depth to estimate the genome size [49][50]
- estimate RNA-seq level [51]
Pseudocode
Determining the possible k-mers of a read can be done by simply cycling over the string length by one and taking out each substring of length, k. The pseudocode to achieve this is as follows:
procedure k-mer(String, k : length of each k-mer)
n = length(String)
/* cycle over the length of String till k-mers of length, k, can still be made */
for i = 1 to n-k+1 inclusive do
/* output each k-mer of length k, from i to i+k in String*/
output String[i:i+k]
end for
end procedure
Examples
Here are some examples showing the possible k-mers (given a specified k value) from DNA sequences:
Read: AGATCGAGTG 3-mers: AGA GAT ATC TCG CGA GAG AGT GTG
Read: GTAGAGCTGT 5-mers: GTAGA TAGAG AGAGC GAGCT AGCTG GCTGT
References
- ^ Compeau, Phillip E C; Pevzner, Pavel A; Tesler, Glenn (2011-11). "How to apply de Bruijn graphs to genome assembly". Nature Biotechnology. 29 (11): 987–991. doi:10.1038/nbt.2023. ISSN 1087-0156.
{{cite journal}}: Check date values in:|date=(help) - ^ Welch, Mark; Govindarajan, Sridhar; Ness, Jon E.; Villalobos, Alan; Gurney, Austin; Minshull, Jeremy; Gustafsson, Claes (2009-09-14). Kudla, Grzegorz (ed.). "Design Parameters to Control Synthetic Gene Expression in Escherichia coli". PLoS ONE. 4 (9): e7002. doi:10.1371/journal.pone.0007002. ISSN 1932-6203.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Gustafsson, Claes; Govindarajan, Sridhar; Minshull, Jeremy (2004-7). "Codon bias and heterologous protein expression". Trends in Biotechnology. 22 (7): 346–353. doi:10.1016/j.tibtech.2004.04.006.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Perry, Scott C.; Beiko, Robert G. (2010-01-01). "Distinguishing Microbial Genome Fragments Based on Their Composition: Evolutionary and Comparative Genomic Perspectives". Genome Biology and Evolution. 2: 117–131. doi:10.1093/gbe/evq004. ISSN 1759-6653.
- ^ Eschke, Kathrin; Trimpert, Jakob; Osterrieder, Nikolaus; Kunec, Dusan (2018-01-29). Mocarski, Edward (ed.). "Attenuation of a very virulent Marek's disease herpesvirus (MDV) by codon pair bias deoptimization". PLOS Pathogens. 14 (1): e1006857. doi:10.1371/journal.ppat.1006857. ISSN 1553-7374.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mapleson, Daniel; Garcia Accinelli, Gonzalo; Kettleborough, George; Wright, Jonathan; Clavijo, Bernardo J. (2016-10-22). "KAT: a K-mer analysis toolkit to quality control NGS datasets and genome assemblies". Bioinformatics: btw663. doi:10.1093/bioinformatics/btw663. ISSN 1367-4803.
- ^ Chor, Benny; Horn, David; Goldman, Nick; Levy, Yaron; Massingham, Tim (2009). "Genomic DNA k-mer spectra: models and modalities". Genome Biology. 10 (10): R108. doi:10.1186/gb-2009-10-10-r108. ISSN 1465-6906.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Yakovchuk, P. (2006-01-30). "Base-stacking and base-pairing contributions into thermal stability of the DNA double helix". Nucleic Acids Research. 34 (2): 564–574. doi:10.1093/nar/gkj454. ISSN 0305-1048.
- ^ Bernardi, Giorgio (2000-1). "Isochores and the evolutionary genomics of vertebrates". Gene. 241 (1): 3–17. doi:10.1016/S0378-1119(99)00485-0.
{{cite journal}}: Check date values in:|date=(help) - ^ Hurst, Laurence D.; Merchant, Alexa R. (2001-03-07). "High guanine–cytosine content is not an adaptation to high temperature: a comparative analysis amongst prokaryotes". Proceedings of the Royal Society of London. Series B: Biological Sciences. 268 (1466): 493–497. doi:10.1098/rspb.2000.1397. ISSN 1471-2954.
- ^ Mugal, Carina F.; Weber, Claudia C.; Ellegren, Hans (2015-12). "GC-biased gene conversion links the recombination landscape and demography to genomic base composition: GC-biased gene conversion drives genomic base composition across a wide range of species". BioEssays. 37 (12): 1317–1326. doi:10.1002/bies.201500058.
{{cite journal}}: Check date values in:|date=(help) - ^ Mugal, Carina F.; Weber, Claudia C.; Ellegren, Hans (2015-12). "GC-biased gene conversion links the recombination landscape and demography to genomic base composition: GC-biased gene conversion drives genomic base composition across a wide range of species". BioEssays. 37 (12): 1317–1326. doi:10.1002/bies.201500058.
{{cite journal}}: Check date values in:|date=(help) - ^ Romiguier, Jonathan; Roux, Camille (2017-02-15). "Analytical Biases Associated with GC-Content in Molecular Evolution". Frontiers in Genetics. 8. doi:10.3389/fgene.2017.00016. ISSN 1664-8021.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Spencer, C.C.A. (2006-08-01). "Human polymorphism around recombination hotspots: Figure 1". Biochemical Society Transactions. 34 (4): 535–536. doi:10.1042/BST0340535. ISSN 0300-5127.
- ^ Weber, Claudia C; Boussau, Bastien; Romiguier, Jonathan; Jarvis, Erich D; Ellegren, Hans (2014-12). "Evidence for GC-biased gene conversion as a driver of between-lineage differences in avian base composition". Genome Biology. 15 (12). doi:10.1186/s13059-014-0549-1. ISSN 1474-760X.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ Lassalle, Florent; Périan, Séverine; Bataillon, Thomas; Nesme, Xavier; Duret, Laurent; Daubin, Vincent (2015-02-06). Petrov, Dmitri A. (ed.). "GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands". PLOS Genetics. 11 (2): e1004941. doi:10.1371/journal.pgen.1004941. ISSN 1553-7404.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Santoyo, G; Romero, D (2005-4). "Gene conversion and concerted evolution in bacterial genomes". FEMS Microbiology Reviews. 29 (2): 169–183. doi:10.1016/j.femsre.2004.10.004.
{{cite journal}}: Check date values in:|date=(help) - ^ Bhérer, Claude; Auton, Adam (2014-06-16), John Wiley & Sons Ltd (ed.), "Biased Gene Conversion and Its Impact on Genome Evolution", eLS, John Wiley & Sons, Ltd, doi:10.1002/9780470015902.a0020834.pub2, ISBN 9780470015902, retrieved 2019-06-23
- ^ Karlin, Samuel (1998-10). "Global dinucleotide signatures and analysis of genomic heterogeneity". Current Opinion in Microbiology. 1 (5): 598–610. doi:10.1016/S1369-5274(98)80095-7.
{{cite journal}}: Check date values in:|date=(help) - ^ Beutler, E.; Gelbart, T.; Han, J. H.; Koziol, J. A.; Beutler, B. (1989-01-01). "Evolution of the genome and the genetic code: selection at the dinucleotide level by methylation and polyribonucleotide cleavage". Proceedings of the National Academy of Sciences. 86 (1): 192–196. doi:10.1073/pnas.86.1.192. ISSN 0027-8424.
- ^ Di Giallonardo, Francesca; Schlub, Timothy E.; Shi, Mang; Holmes, Edward C. (2017-04-15). Dermody, Terence S. (ed.). "Dinucleotide Composition in Animal RNA Viruses Is Shaped More by Virus Family than by Host Species". Journal of Virology. 91 (8). doi:10.1128/JVI.02381-16. ISSN 0022-538X.
- ^ Żemojtel, Tomasz; kiełbasa, Szymon M.; Arndt, Peter F.; Behrens, Sarah; Bourque, Guillaume; Vingron, Martin (2011-01-01). "CpG Deamination Creates Transcription Factor–Binding Sites with High Efficiency". Genome Biology and Evolution. 3: 1304–1311. doi:10.1093/gbe/evr107. ISSN 1759-6653.
- ^ Karlin, Samuel (1998-10). "Global dinucleotide signatures and analysis of genomic heterogeneity". Current Opinion in Microbiology. 1 (5): 598–610. doi:10.1016/S1369-5274(98)80095-7.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Hershberg R, Petrov DA. Selection on Codon Bias. Annual Review of Genetics. Annual Reviews; 2008;42: 287–299. doi:10.1146/annurev.genet.42.110807.091442
- ^ Sharp, Paul M.; Li, Wen-Hsiung (1987). "The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications". Nucleic Acids Research. 15 (3): 1281–1295. doi:10.1093/nar/15.3.1281. ISSN 0305-1048.
- ^ Noble, Peter A.; Citek, Robert W.; Ogunseitan, Oladele A. (1998-04). "Tetranucleotide frequencies in microbial genomes". Electrophoresis. 19 (4): 528–535. doi:10.1002/elps.1150190412. ISSN 0173-0835.
{{cite journal}}: Check date values in:|date=(help) - ^ Compeau, P.; Pevzner, P.; Teslar, G. (2011). "How to apply de Bruijn graphs to genome assembly". Nature Biotechnology. 29 (11): 987–991. doi:10.1038/nbt.2023. PMC 5531759. PMID 22068540.
- ^ a b Zerbino, Daniel R.; Birney, Ewan (2008). "Velvet: algorithms for de novo short read assembly using de Bruijn graphs". Genome Research. 18 (5): 821–829. doi:10.1101/gr.074492.107. PMC 2336801. PMID 18349386.
- ^ Rachid Ounit; Steve Wanamaker; Timothy J Close; Stefano Lonardi (2015). "CLARK: fast and accurate classification of metagenomic and genomic sequences using discriminative k-mers". BMC Genomics. 16: 236. doi:10.1186/s12864-015-1419-2. PMC 4428112. PMID 25879410.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Dubinkina; Ischenko; Ulyantsev; Tyakht; Alexeev (2016). "Assessment of k-mer spectrum applicability for metagenomic dissimilarity analysis". BMC Bioinformatics. 17: 38. doi:10.1186/s12859-015-0875-7. PMC 4715287. PMID 26774270.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Zhu, Zheng (2014). "Self-organizing approach for meta-genomes". Computational Biology and Chemistry. 53: 118–124. doi:10.1016/j.compbiolchem.2014.08.016. PMID 25213854.
- ^ Chor; Horn; Goldman; Levy; Massingham (2009). "Genomic DNA k-mer spectra: models and modalities". Genome Biology. 10 (10): R108. doi:10.1186/gb-2009-10-10-r108. PMC 2784323. PMID 19814784.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Meher, Sahu, Rao (2016). "Identification of species based on DNA barcode using k-mer feature vector and Random forest classifier". Gene. 592 (2): 316–324. doi:10.1016/j.gene.2016.07.010. PMID 27393648.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li; et al. (2010). "De novo assembly of human genomes with massively parallel short read sequencing". Genome Research. 20 (2): 265–272. doi:10.1101/gr.097261.109. PMC 2813482. PMID 20019144.
- ^ Navarro-Gomez; et al. (2015). "Phy-Mer: a novel alignment-free and reference-independent mitochondrial haplogroup classifier". Bioinformatics. 31 (8): 1310–1312. doi:10.1093/bioinformatics/btu825. PMC 4393525. PMID 25505086.
- ^ Phillippy, Schatz, Pop (2008). "Genome assembly forensics: finding the elusive mis-assembly". Bioinformatics. 9 (3): R55. doi:10.1186/gb-2008-9-3-r55. PMC 2397507. PMID 18341692.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Price, Jones, Pevzner (2005). "De novo identification of repeat families in large genomes". Bioinformatics. 21(supp 1): i351–8. doi:10.1093/bioinformatics/bti1018. PMID 15961478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Newburger, Bulyk (2009). "UniPROBE: an online database of protein binding microarray data on protein–DNA interactions". Nucleic Acids Research. 37(supp 1) (Database issue): D77–82. doi:10.1093/nar/gkn660. PMC 2686578. PMID 18842628.
- ^ S. Burkhardt and J. Kärkkäinen (2002). Better filtering with gapped q-grams. Lecture Notes in Computer Science. Vol. 56. pp. 51–70. CiteSeerX 10.1.1.57.8820. doi:10.1007/3-540-45452-7_19. ISBN 978-3-540-43862-5.
{{cite book}}:|journal=ignored (help) - ^ Keich; et al. (2004). "On spaced seeds for similarity search". Discrete Applied Mathematics. 138 (3): 253–263. doi:10.1016/S0166-218X(03)00382-2.
- ^ Ghandi; et al. (2014). "Enhanced regulatory sequence prediction using gapped k-mer features". PLoS Computational Biology. 10 (7): e1003711. Bibcode:2014PLSCB..10E3711G. doi:10.1371/journal.pcbi.1003711. PMC 4102394. PMID 25033408.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nordstrom; et al. (2013). "Mutation identification by direct comparison of whole-genome sequencing data from mutant and wild-type individuals using k-mers". Nature Biotechnology. 31 (4): 325–330. doi:10.1038/nbt.2515. PMID 23475072.
- ^ Chae; et al. (2013). "Comparative analysis using K-mer and K-flank patterns provides evidence for CpG island sequence evolution in mammalian genomes". Nucleic Acids Research. 41 (9): 4783–4791. doi:10.1093/nar/gkt144. PMC 3643570. PMID 23519616.
- ^ Mohamed Hashim, Abdullah (2015). "Rare k-mer DNA: Identification of sequence motifs and prediction of CpG island and promoter". Journal of Theoretical Biology. 387: 88–100. doi:10.1016/j.jtbi.2015.09.014. PMID 26427337.
- ^ Jaron, Moravec, Martinkova (2014). "SigHunt: horizontal gene transfer finder optimized for eukaryotic genomes". Bioinformatics. 30 (8): 1081–1086. doi:10.1093/bioinformatics/btt727. PMID 24371153.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Delmont, Eren (2016). "Identifying contamination with advanced visualization and analysis practices: metagenomic approaches for eukaryotic genome assemblies". PeerJ. 4: e1839. doi:10.7717/Fpeerj.1839 (inactive 2019-05-24).
{{cite journal}}: CS1 maint: DOI inactive as of May 2019 (link) CS1 maint: unflagged free DOI (link) - ^ Bemm; et al. (2016). "Genome of a tardigrade: Horizontal gene transfer or bacterial contamination?". Proceedings of the National Academy of Sciences. 113 (22): E3054–E3056. doi:10.1073/pnas.1525116113. PMC 4896698. PMID 27173902.
- ^ Wang, Xu, Liu (2016). "Recombination spot identification Based on gapped k-mers". Scientific Reports. 6: 23934. Bibcode:2016NatSR...623934W. doi:10.1038/srep23934. PMC 4814916. PMID 27030570.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hozza, Vinar, Brejova (2015). How big is that genome? estimating genomesize and coverage from k-mer abundance spectra. SPIRE 2015. doi:10.1007/978-3-319-23826-5_20.
{{cite conference}}: CS1 maint: multiple names: authors list (link) - ^ Lamichhaney; et al. (2016). "Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax)". Nature Genetics. 48 (1): 84–88. doi:10.1038/ng.3430. PMID 26569123.
- ^ Patro, Mount, Kingsford (2014). "Sailfish enables alignment-free isoform quantification from RNA-seq reads using lightweight algorithms". Nature Biotechnology. 32 (5): 462–464. arXiv:1308.3700. doi:10.1038/nbt.2862. PMC 4077321. PMID 24752080.
{{cite journal}}: CS1 maint: multiple names: authors list (link)







