Lysenin: Difference between revisions
No edit summary |
implementing updates from v:WikiJournal_of_Science/Lysenin, omitting preprint refs and figure numbers |
||
| Line 3: | Line 3: | ||
==Monomer== |
==Monomer== |
||
[[File:Lysenin monomer.tif|thumb| |
[[File:Lysenin monomer.tif|thumb|Lysenin water-soluble monomeric X-ray structure ({{PDB|3ZXD}}). Receptor binding domain on right in grey. Pore Forming Module (PFM) on left with region ''previously'' though to be responsible for β-barrel formation in green. Additional region ''now'' known to be important in β-barrel formation in yellow (from X-ray data),]] |
||
Lysenin is a [[ |
Lysenin is a [[protein]] produced in the [[coelomocyte]]-[[leucocyte|leucocytes]] of the earthworm ''[[Eisenia fetida]]''.<ref>{{cite journal|last1=Yilmaz|first1=N.|last2=Yamaji-Hasegawa|first2=A.|last3=Hullin-Matsuda|first3=F.|last4=Kobayashi|first4=T.|date=2018|title=Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin|journal=Seminars in Cell & Developmental Biology|language=English|volume=73|pages=188–198|doi=10.1016/j.semcdb.2017.07.036}}</ref> This protein was first isolated from the coelomic fluid in 1996 and named lysenin (from lysis and ''Eisenia'').<ref>{{cite journal|last1=Sekizawa|first1=Y.|last2=Hagiwara|first2=K.|last3=Nakajima|first3=T.|last4=Kobayashi|first4=H.|date=1996|title=A Novel Protein, Lysenin, that Causes Contraction of the Isolate Rat Aorta: Its Purification from the Coelomic Fluid of the Earthworm, ''Eisenia foetida''|journal=Biomedical Research|language=English|volume=17|issue=3|pages=197–203|doi=10.2220/biomedres.17.197}}</ref> Lysenin is a relatively small water-soluble molecule with a molecular weight of 33 kDa. Using [[X-ray crystallography]], lysenin was classified as a member of the [[Aerolysin]] protein family by structure and function.<ref name="Colibus2">{{cite journal|last1=De Colibus|first1=L.|last2=Sonnen|first2=A. F.-P.|last3=Morris|first3=K. J.|last4=Siebert|first4=C. A.|last5=Abrusci|first5=P.|last6=Plitzko|first6=J.|last7=Hodnik|first7=V.|last8=Leippe|first8=M.|last9=Volpi|first9=E.|date=2012|title=Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition|journal=Structure|language=English|volume=20|issue=9|pages=1498–1507|doi=10.1016/j.str.2012.06.011|last10=Anderluh|first10=G.|last11=Gilbert|first11=R. J. C.}}</ref> Structurally, each lysenin monomer consists of a receptor binding domain (grey globular part on right of Figure 1) and a Pore Forming Module (PFM); domains shared throughout the aerolysin family.<ref name="Colibus2" /> The lysenin receptor binding domain shows three [[sphingomyelin]] binding motifs. The Pore Forming Module contains the regions that undergo large conformational changes to become the β-barrel in the pore.<ref name="Bokori2">{{cite journal|last1=Bokori-Brown|first1=M.|last2=Martin|first2=T. G.|last3=Naylor|first3=C. E.|last4=Basak|first4=A. K.|last5=Titball|first5=R. W.|last6=Savva|first6=C. G.|date=2016|title=Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein|journal=Nature Communications|language=English|volume=7|issue=1|doi=10.1038/ncomms11293}}</ref> |
||
==Membrane receptors== |
==Membrane receptors== |
||
The natural [[ |
The natural [[Cell membrane|membrane]] target of lysenin is an animal plasma membrane [[lipid]] called [[sphingomyelin]] located mainly in its outer leaflet, involving at least three of its [[Phosphatidylcholine|phosphatidylcholines]] (PC) groups.<ref name=":0">{{cite journal|last1=Ishitsuka|first1=R.|last2=Kobayashi|first2=T.|date=2007|title=Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin|journal=Biochemistry|language=English|volume=46|issue=6|pages=1495–1502|doi=10.1021/bi061290k}}</ref> Sphingomyelin is usually found associated with [[cholesterol]] in [[Lipid raft|lipid rafts]].<ref>{{cite journal|last1=Simons|first1=K.|last2=Gerl|first2=M. J.|date=2010|title=Revitalizing membrane rafts: new tools and insights|journal=Nature Reviews Molecular Cell Biology|language=English|volume=11|issue=10|pages=688–699|doi=10.1038/nrm2977}}</ref> Cholesterol, which enhances [[oligomerization]], provides a stable platform with high lateral mobility where monomer-monomer encounters are more probable.<ref name=":0" /> PFTs have shown to be able to remodel the membrane structure,<ref name="More2">{{cite journal|last1=Ros|first1=U.|last2=García-Sáez|first2=A. J.|date=2015|title=More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes|journal=The Journal of Membrane Biology|language=English|volume=248|issue=3|pages=545–561|doi=10.1007/s00232-015-9820-y}}</ref> sometimes even mixing lipid phases.<ref>{{cite journal|last1=Yilmaz|first1=N.|last2=Kobayashi|first2=T.|date=2015|title=Visualization of Lipid Membrane Reorganization Induced by a Pore-Forming Toxin Using High-Speed Atomic Force Microscopy|journal=ACS Nano|language=English|volume=9|issue=8|pages=7960–7967|doi=10.1021/acsnano.5b01041}}</ref> |
||
PFTs have shown to be able to remodel the membrane structure,<ref name=More>Ros, U. & García-Sáez, A. J. More than a pore: the interplay of pore-forming proteins and lipid membranes. The Journal of membrane biology 248, 545–561 (2015).</ref> sometimes even mixing lipid phases.<ref>Yilmaz, N. & Kobayashi, T. Visualization of lipid membrane reorganization induced by a pore-forming toxin using high-speed atomic force microscopy. ACS nano 9, 7960–7967 (2015).</ref> In lysenin, the detergent belt is 32 Å in height. The detergent belt is the part of the β-barrel occupied by detergent in Cryogenic Electron Microscopy (Cryo-EM) studies of Lysenin pore,therefore, is the part of the β-barrel inmersed in the hydrophobic region of the membrane,<ref name=Bokori />. On the other hand, sphingomyelin/Cholesterol bilayers are about 4.5 nm height.<ref>Quinn, P. J. Structure of sphingomyelin bilayers and complexes with cholesterol forming membrane rafts. Langmuir 29, 9447–9456 (2013).</ref> This difference in height between the detergent belt and the sphingomyelin/cholesterol bilayer implies a bend of the membrane in the region surrounding the pore, called negative mismatch.<ref>Guigas, G. & Weiss, M. Effects of protein crowding on membrane systems. Biochimica et Biophysica Acta (BBA)-Biomembranes 1858, 2441–2450 (2016).</ref> This bending results in a net attraction between pores that induce pores aggregation.<ref name=crowd>Ignacio L.B. Munguira, Alfonso Barbas, and ignacio Casuso. Mechanism of Blocking and Unblocking of the Pore Formation of the Toxin Lysenin Regulated by Local Crowding. Under Submision.</ref> |
|||
The region of the lysenin pore β-barrel expected to be inmersed in the hydrophobic region of the membrane is the 'detergent belt', the 3.2 nm high region occupied by detergent in [[Cryogenic Electron Microscopy]] (Cryo-EM) studies of the pore.<ref name="Bokori3">{{cite journal|last1=Bokori-Brown|first1=M.|last2=Martin|first2=T. G.|last3=Naylor|first3=C. E.|last4=Basak|first4=A. K.|last5=Titball|first5=R. W.|last6=Savva|first6=C. G.|date=2016|title=Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein|journal=Nature Communications|language=English|volume=7|issue=1|doi=10.1038/ncomms11293}}</ref> On the other hand, sphingomyelin/Cholesterol bilayers are about 4.5 nm height.<ref>{{cite journal|last1=Quinn|first1=P. J.|date=2013|title=Structure of Sphingomyelin Bilayers and Complexes with Cholesterol Forming Membrane Rafts|journal=Langmuir|language=English|volume=29|issue=30|pages=9447–9456|doi=10.1021/la4018129}}</ref> This difference in height between the detergent belt and the sphingomyelin/cholesterol bilayer implies a bend of the membrane in the region surrounding the pore, called negative mismatch.<ref>{{cite journal|last1=Guigas|first1=G.|last2=Weiss|first2=M.|date=2016|title=Effects of protein crowding on membrane systems|journal=Biochimica et Biophysica Acta (BBA) - Biomembranes|language=English|volume=1858|issue=10|pages=2441–2450|doi=10.1016/j.bbamem.2015.12.021}}</ref> This bending results in a net attraction between pores that induce pores aggregation. |
|||
==Binding, oligomerization and insertion== |
==Binding, oligomerization and insertion== |
||
[[File:Lysenin action mechanism.png|thumb|Lysenin mechanism of action Scheme. '''a)''' Lysenin monomers are segregated as soluble proteins that bind specifically to sphingomyelin by its receptor binding domain. After binding, and reach a certain density, the oligomerization starts. '''b)''' After a complete oligomerization, the prepore is formed. The prepore model shown here was assembled from the monomer structure and aligned with the pore structure ({{PDB|5GAQ}}) by their receptor-binding domains (residues 160 to 297). The height of the prepore was set to agree with the Atomic Force Microscopy measurements. '''c)''' Membrane inserted Lysenin assembly ({{PDB|5GAQ}}). The height of the pore was measured from the detergent belt to the last residue, assuming that the detergent belt corresponds with the part of the pore surrounded by the membrane. The membrane was placed in the β-barrel of the pore to match with the detergent belt, that englobe all the hydrophobic residues of the β-barrel. The hydrophobic surface colour scale is according to the hydrophobicity scale of Kyte and Doolittle.|alt=|400x400px]]Membrane binding is a requisite to initiate PFT oligomerization. Lysenin monomers bind specifically to sphingomyelin via the receptor binding domain.<ref name="Colibus3">{{cite journal|last1=De Colibus|first1=L.|last2=Sonnen|first2=A. F.-P.|last3=Morris|first3=K. J.|last4=Siebert|first4=C. A.|last5=Abrusci|first5=P.|last6=Plitzko|first6=J.|last7=Hodnik|first7=V.|last8=Leippe|first8=M.|last9=Volpi|first9=E.|date=2012|title=Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition|journal=Structure|language=English|volume=20|issue=9|pages=1498–1507|doi=10.1016/j.str.2012.06.011|last10=Anderluh|first10=G.|last11=Gilbert|first11=R. J. C.}}</ref> The final lysenin oligomer is constituted by nine monomers without quantified deviations.<ref>{{cite journal|last1=Munguira|first1=I.|last2=Casuso|first2=I.|last3=Takahashi|first3=H.|last4=Rico|first4=F.|last5=Miyagi|first5=A.|last6=Chami|first6=M.|last7=Scheuring|first7=S.|date=2016|title=Glasslike Membrane Protein Diffusion in a Crowded Membrane|journal=ACS Nano|language=English|volume=10|issue=2|pages=2584–2590|doi=10.1021/acsnano.5b07595}}</ref> When lysenin monomers bind to sphingomyelin-enriched membrane regions, they provide a stable platform with a high lateral mobility, hence favouring the oligomerization.<ref name=":02">{{cite journal|last1=Ishitsuka|first1=R.|last2=Kobayashi|first2=T.|date=2007|title=Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin|journal=Biochemistry|language=English|volume=46|issue=6|pages=1495–1502|doi=10.1021/bi061290k}}</ref> As with most PFTs, lysenin oligomerization occurs in a two-step process, as was recently imaged. |
|||
| ⚫ | The process begins with monomers being adsorbed into the membrane by specific interactions, resulting in an increased concentration of monomers. This increase is promoted by the small area where the membrane receptor accumulates owing to the fact that the majority of PFT membrane receptors are associated with lipid rafts.<ref>{{cite journal|last1=Lafont|first1=F.|last2=Van Der Goot|first2=F. G.|date=2005|title=Bacterial invasion via lipid rafts|journal=Cellular Microbiology|language=English|volume=7|issue=5|pages=613–620|doi=10.1111/j.1462-5822.2005.00515.x}}</ref> Another side effect, aside from the increase of monomer concentration, is the monomer-monomer interaction. This interaction increases lysenin oligomerization. After a critical threshold concentration is reached, several oligomers are formed simultaneously, although sometimes these are incomplete.<ref name="yfirst2">{{cite journal|last1=Yilmaz|first1=N.|last2=Yamada|first2=T.|last3=Greimel|first3=P.|last4=Uchihashi|first4=T.|last5=Ando|first5=T.|last6=Kobayashi|first6=T.|date=2013|title=Real-Time Visualization of Assembling of a Sphingomyelin-Specific Toxin on Planar Lipid Membranes|journal=Biophysical Journal|language=English|volume=105|issue=6|pages=1397–1405|doi=10.1016/j.bpj.2013.07.052}}</ref> In contrast to PFTs of the [[cholesterol-dependent cytolysin]] family,<ref>{{cite journal|last1=Mulvihill|first1=E.|last2=van Pee|first2=K.|last3=Mari|first3=S. A.|last4=Müller|first4=D. J.|last5=Yildiz|first5=Ö.|date=2015|title=Directly Observing the Lipid-Dependent Self-Assembly and Pore-Forming Mechanism of the Cytolytic Toxin Listeriolysin O|journal=Nano Letters|language=English|volume=15|issue=10|pages=6965–6973|doi=10.1021/acs.nanolett.5b02963}}</ref> the transition from incomplete lysenin oligomers to complete oligomers has not been observed. |
||
Membrane binding is a requisite to initiate PFT oligomerization. Lysenin monomers bind specifically to sphingomyelin via the receptor binding domain.<ref name=Colibus /> The final Lysenin oligomer is constituted by nine monomers without quantified deviations''.''<ref>Munguira, I. et al. Glasslike Membrane Protein Diffusion in a Crowded Membrane. ACS Nano 10, 2584–2590 (2016).</ref> When Lysenin monomers bind to sphingomyelin-enriched domains, they provide a stable platform with a high lateral mobility, hence favouring the oligomerization.<ref>Ishitsuka, R. & Kobayashi, T. Cholesterol and lipid/protein ratio control the oligomerization of a sphingomyelin-specific toxin, lysenin. Biochemistry 46, 1495–1502 (2007).</ref> Like most proteins, Lysenin oligomerization occurs in a two-step process, as was recently imaged.<ref name=crowd></ref> |
|||
| ⚫ | A complete oligomerization results in the so-called prepore state, a structure on the membrane. Determining the prepore's structure by X-ray or Cryo-EM is a challenging process that so far has not produced any results. The only available information about the prepore structure was provided by [[Atomic force microscopy|Atomic Force Microscopy]] (AFM). The measured prepore height was 90 Å; and the width 118 Å, with an inner pore of 50 Å.<ref name="yfirst2" /> A model of the prepore was built aligning the monomer structure ({{PDB|3ZXD}}) with the pore structure ({{PDB|5GAQ}}) by their receptor-binding domains (residues 160 to 297). A recent study in aerolysin suggests that the currently accepted model for the lysenin prepore should be revisited, according to the new available data on the aerolysin insertion. |
||
| ⚫ | The process begins with monomers being adsorbed into the membrane by specific interactions, resulting in an increased concentration of monomers. This increase is promoted by the small area where the membrane receptor accumulates owing to the fact that the majority of PFT membrane receptors are associated with lipid rafts.<ref> |
||
| ⚫ | A [[conformational change]] transforms the PFM into the transmembrane [[β-barrel]], leading to the pore state.<ref name="Bokori4">{{cite journal|last1=Bokori-Brown|first1=M.|last2=Martin|first2=T. G.|last3=Naylor|first3=C. E.|last4=Basak|first4=A. K.|last5=Titball|first5=R. W.|last6=Savva|first6=C. G.|date=2016|title=Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein|journal=Nature Communications|language=English|volume=7|issue=1|doi=10.1038/ncomms11293}}</ref> The trigger mechanism for the prepore-to-pore transition in lysenin depends on three glutamic acid residues (E92, E94 and E97), and is activated by a decrease in pH,<ref>{{cite journal|last1=Munguira|first1=I. L. B.|last2=Takahashi|first2=H.|last3=Casuso|first3=I.|last4=Scheuring|first4=S.|date=2017|title=Lysenin Toxin Membrane Insertion Is pH-Dependent but Independent of Neighboring Lysenins|journal=Biophysical Journal|language=English|volume=113|issue=9|pages=2029–2036|doi=10.1016/j.bpj.2017.08.056}}</ref> from physiological conditions to the acidic conditions reached after endocytosis. These three glutamic acids are located in an α-helix that forms part of the PFM, and glutamic acids are found in aerolysin family members in its PFMs. Such a conformational change produces a decrease in the oligomer height of 2.5 nm according to AFM measurements.<ref name="yfirst2" /> The main dimensions, using lysenin pore X-ray structure, are height 97 Å, width 115 Å and the inner pore of 30 Å.<ref name="Bokori4" /> However, complete oligomerization into the nonamer is not a requisite for the insertion, since incomplete oligomers in the pore state can be found.<ref name="yfirst2" /> The prepore to pore transition can be blocked in crowded conditions, a mechanism that could be general to all β-PFTs. The first hint of crowding effect on prepore to pore transition was given by congestion effects in electrophysiology experiments.<ref>{{cite journal|last1=Krueger|first1=E.|last2=Bryant|first2=S.|last3=Shrestha|first3=N.|last4=Clark|first4=T.|last5=Hanna|first5=C.|last6=Pink|first6=D.|last7=Fologea|first7=D.|date=2015|title=Intramembrane congestion effects on lysenin channel voltage-induced gating|journal=European Biophysics Journal|language=English|volume=45|issue=2|pages=187–194|doi=10.1007/s00249-015-1104-z}}</ref> High-Speed AFM studies incubating lysenin on sphingomyelin/cholesterol membranes has shown that under crowded conditions the prepore to pore transition gets blocked by steric interactions.<ref>{{cite thesis|first1=I. L. B.|last1=Munguira|title=Effect of Crowdedness in the Life Cycle of Lysenin Studied by High-Speed Atomic Force Microscopy|publisher=Aix-Marseille Universite|date=2017|url=http://www.theses.fr/2017AIXM0124|type=PhD|language=English}}</ref> |
||
| ⚫ | A complete oligomerization results in the so-called prepore state, a structure on the membrane. Determining the prepore's structure by X-ray or Cryo-EM is a challenging process that so far has not produced any results. The only available information about the prepore structure was provided by [[ |
||
[[File:Lysenin action mechanism.png|thumb|Figure 2. Lysenin mechanism of action Scheme. a) Lysenin monomers are segregated as soluble proteins that bind specifically to sphingomyelin by its receptor binding domain. After binding, and reach a certain density, the oligomerization starts. b) After a complete oligomerization, the prepore is formed. The prepore model shown here was assembled from the monomer structure and aligned with the pore structure (PDB ID 5GAQ) by their receptor-binding domains (residues 160 to 297). The height of the prepore was set to agree with the Atomic Force Microscopy measurements. c) Membrane inserted Lysenin assembly (PDB ID 5GAQ). The height of the pore was measured from the detergent belt to the last residue, assuming that the detergent belt corresponds with the part of the pore surrounded by the membrane. The membrane was placed in the β-barrel of the pore to match with the detergent belt, that englobe all the hydrophobic residues of the β-barrel. The hydrophobic surface colour scale is according to the hydrophobicity scale of Kyte and Doolittle.]] |
|||
| ⚫ | A conformational change transforms the PFM |
||
==Insertion consequences== |
==Insertion consequences== |
||
| ⚫ | |||
* Breaking the sphingomyelin asymmetry between the two leaflets of the lipid bilayer by punching holes in the membrane<ref>{{cite journal|last1=Green|first1=D. R.|date=2000|title=Apoptosis and Sphingomyelin Hydrolysis|journal=The Journal of Cell Biology|language=English|volume=150|issue=1|pages=F5–F8|doi=10.1083/jcb.150.1.F5}}</ref> and inducing [[lipid flip-flop]] (reorientation of a lipid from one leaflet of a membrane bilayer to the other).<ref name="More4">{{cite journal|last1=Ros|first1=U.|last2=García-Sáez|first2=A. J.|date=2015|title=More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes|journal=The Journal of Membrane Biology|language=English|volume=248|issue=3|pages=545–561|doi=10.1007/s00232-015-9820-y}}</ref> |
|||
| ⚫ | |||
| ⚫ | * Increasing the calcium concentration in the cytoplasm.<ref>{{cite journal|last1=Orrenius|first1=S.|last2=Zhivotovsky|first2=B.|last3=Nicotera|first3=P.|date=2003|title=Regulation of cell death: the calcium–apoptosis link|journal=Nature Reviews Molecular Cell Biology|language=English|volume=4|issue=7|pages=552–565|doi=10.1038/nrm1150}}</ref> |
||
| ⚫ | * Decreasing the potassium concentration in the cytoplasm.<ref>{{cite journal|last1=Yu|first1=S. P.|date=2003|title=Regulation and critical role of potassium homeostasis in apoptosis|journal=Progress in Neurobiology|language=English|volume=70|issue=4|pages=363–386|doi=10.1016/s0301-0082(03)00090-x}}</ref> |
||
* Punching the membrane breaks the sphingomyelin asymmetry between the two leaflets of the lipid bilayer, which cause [[w:apoptosis|apoptosis]] of the cell.<ref>Green, D. R. Apoptosis and sphingomyelin hydrolysis: the flip side. J Cell Biol 150, F5–F8 (2000).</ref> PFTs are supposed to induce lipid flip-flop (reorientation of a lipid from one leaflet of a membrane bilayer to the other) that can also break the sphingomyelin asymmetry.<ref name=More /> |
|||
| ⚫ | |||
| ⚫ | |||
==Biological role== |
==Biological role== |
||
The biological role of lysenin remains unknown. It |
The biological role of lysenin remains unknown. It has been suggested that lysenin may play a role as a [[anti-predator adaptation|defence mechanism]] against attackers such as [[bacteria]], [[fungi]] or small [[invertebrates]].<ref>{{cite book|title=Lessons in immunity: from single-cell organisms to mammals|last1=Ballarin|first1=L.|last2=Cammarata|first2=M.|date=2016|publisher=Academic Press|isbn=9780128032527}}</ref> However, lysenin's activity is dependent upon binding to sphingomyelin, which is not present in the membranes of bacteria, fungi or most invertebrates. Rather, sphingomyelin is mainly present in the plasma membrane of [[chordates]].<ref>{{cite journal|last1=Kobayashi|first1=H.|last2=Sekizawa|first2=Y.|last3=Aizu|first3=M.|last4=Umeda|first4=M.|date=2000|title=Lethal and non-lethal responses of spermatozoa from a wide variety of vertebrates and invertebrates to lysenin, a protein from the coelomic fluid of the earthworm ''Eisenia foetida''|journal=Journal of Experimental Zoology|language=English|volume=286|issue=5|pages=538–549|doi=10.1002/(sici)1097-010x(20000401)286:5<538::aid-jez12>3.0.co;2-w}}</ref> Another hypothesis is that the earthworm, which is able to expel coelomic fluid under stress,<ref>{{cite journal|last1=Sukumwang|first1=N.|last2=Umezawa|first2=K.|date=2013|title=Earthworm-Derived Pore-Forming Toxin Lysenin and Screening of Its Inhibitors|journal=Toxins|language=English|volume=5|issue=8|pages=1392–1401|doi=10.3390/toxins5081392}}</ref><ref>{{Cite journal|last=Kobayashi|first=H.|last2=Ohta|first2=N.|last3=Umeda|first3=M.|date=2004|title=Biology of lysenin, a protein in the coelomic fluid of the earthworm ''Eisenia foetida''|url=https://www.ncbi.nlm.nih.gov/pubmed/15261736|journal=International Review of Cytology|volume=236|pages=45–99|doi=10.1016/S0074-7696(04)36002-X|pmid=15261736}}</ref> generates an avoidance behaviour to its [[vertebrate]] predators (such as birds, [[hedgehog|hedgehogs]] or [[Mole (animal)|moles]]).<ref>{{cite journal|last1=Swiderska|first1=B.|last2=Kedracka-Krok|first2=S.|last3=Panz|first3=T.|last4=Morgan|first4=A. J.|last5=Falniowski|first5=A.|last6=Grzmil|first6=P.|last7=Plytycz|first7=B.|date=2017|title=Lysenin family proteins in earthworm coelomocytes – Comparative approach|journal=Developmental & Comparative Immunology|language=English|volume=67|pages=404–412|doi=10.1016/j.dci.2016.08.011}}</ref> If that is the case, the expelled lysenin might be more effective if the coelomic fluid reaches the eye, where the concentration of sphingomyelin is ten times higher than in other body organs.<ref>{{cite book|title=Biochemistry of the Eye|last1=Berman|first1=E. R.|date=1991|publisher=Springer|isbn=978-1-4757-9441-0|language=English|doi=10.1007/978-1-4757-9441-0}}</ref> A complementary hypothesis is that the pungent smell of the coelomic fluid - giving the earthworm its specific epithet ''foetida'' - is an [[anti-predator adaptation]]. However, it remains unknown whether lysenin contributes to avoidance of ''Eisenia'' by predators.<ref>{{cite book|title=Biology and Ecology of Earthworms|last1=Edwards|first1=C. A.|last2=Bohlen|first2=P. J.|date=1996|publisher=Springer Science & Business Media|isbn=978-0-412-56160-3|language=English}}</ref> |
||
==Applications== |
==Applications== |
||
Lysenin conductive properties have been studied for years. |
Lysenin's conductive properties have been studied for years.<ref>{{cite journal|last1=Bryant|first1=S.|last2=Clark|first2=T.|last3=Thomas|first3=C.|last4=Ware|first4=K.|last5=Bogard|first5=A.|last6=Calzacorta|first6=C.|last7=Prather|first7=D.|last8=Fologea|first8=D.|date=2018|title=Insights into the Voltage Regulation Mechanism of the Pore-Forming Toxin Lysenin|journal=Toxins|language=English|volume=10|issue=8|pages=334|doi=10.3390/toxins10080334}}</ref> Like most pore-forming toxins, lysenin forms a non-specific channel that is permeable to ions, small molecules, and small peptides.<ref>{{cite journal|last1=Shrestha|first1=N.|last2=Bryant|first2=S. L.|last3=Thomas|first3=C.|last4=Richtsmeier|first4=D.|last5=Pu|first5=X.|last6=Tinker|first6=J.|last7=Fologea|first7=D.|date=2017|title=Stochastic sensing of Angiotensin II with lysenin channels|journal=Scientific Reports|language=English|volume=7|issue=1|doi=10.1038/s41598-017-02438-0}}</ref> There have also been over three decades of studies into finding suitable pores for converting into [[Nanopore sequencing|nanopore sequencing systems]] that can have their conductive properties tuned by point mutation.<ref>{{cite journal|last1=Deamer|first1=D.|last2=Akeson|first2=M.|last3=Branton|first3=D.|date=2016|title=Three decades of nanopore sequencing|journal=Nature Biotechnology|language=English|volume=34|issue=5|pages=518–524|doi=10.1038/nbt.3423}}</ref> Owing to its binding affinity for sphingomyelin, lysenin (or just the receptor binding domain) has been used as a fluorescence marker to detect the sphingomyelin domain in membranes.<ref>{{cite journal|last1=Ishitsuka|first1=R.|last2=Kobayashi|first2=T.|date=2004|title=Lysenin: A new tool for investigating membrane lipid organization|journal=Anatomical Science International|language=English|volume=79|issue=4|pages=184–190|doi=10.1111/j.1447-073x.2004.00086.x}}</ref> |
||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
== External links == |
|||
* https://www.theses.fr/2017AIXM0124 |
|||
[[Category:Protein toxins]] |
[[Category:Protein toxins]] |
||
Revision as of 03:25, 18 August 2019
Lysenin is a pore-forming toxin (PFT) in the coelomic fluid of the earthworm Eisenia fetida. Pore-forming toxins are a group of proteins that act as virulence factors of several pathogenic bacteria. Following the general mechanism of action of PFTs lysenin is segregated as a soluble monomer that binds specifically to a membrane receptor, sphingomyelin in the case of lysenin. After attaching to the membrane, the oligomerization begins, resulting in a nonamer on top of membrane, known as a prepore. After a conformational change, which could be triggered by a decrease of pH, the oligomer is inserted into the membrane in the so-called pore state.
Monomer
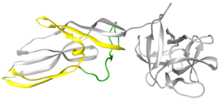
Lysenin is a protein produced in the coelomocyte-leucocytes of the earthworm Eisenia fetida.[1] This protein was first isolated from the coelomic fluid in 1996 and named lysenin (from lysis and Eisenia).[2] Lysenin is a relatively small water-soluble molecule with a molecular weight of 33 kDa. Using X-ray crystallography, lysenin was classified as a member of the Aerolysin protein family by structure and function.[3] Structurally, each lysenin monomer consists of a receptor binding domain (grey globular part on right of Figure 1) and a Pore Forming Module (PFM); domains shared throughout the aerolysin family.[3] The lysenin receptor binding domain shows three sphingomyelin binding motifs. The Pore Forming Module contains the regions that undergo large conformational changes to become the β-barrel in the pore.[4]
Membrane receptors
The natural membrane target of lysenin is an animal plasma membrane lipid called sphingomyelin located mainly in its outer leaflet, involving at least three of its phosphatidylcholines (PC) groups.[5] Sphingomyelin is usually found associated with cholesterol in lipid rafts.[6] Cholesterol, which enhances oligomerization, provides a stable platform with high lateral mobility where monomer-monomer encounters are more probable.[5] PFTs have shown to be able to remodel the membrane structure,[7] sometimes even mixing lipid phases.[8]
The region of the lysenin pore β-barrel expected to be inmersed in the hydrophobic region of the membrane is the 'detergent belt', the 3.2 nm high region occupied by detergent in Cryogenic Electron Microscopy (Cryo-EM) studies of the pore.[9] On the other hand, sphingomyelin/Cholesterol bilayers are about 4.5 nm height.[10] This difference in height between the detergent belt and the sphingomyelin/cholesterol bilayer implies a bend of the membrane in the region surrounding the pore, called negative mismatch.[11] This bending results in a net attraction between pores that induce pores aggregation.
Binding, oligomerization and insertion

Membrane binding is a requisite to initiate PFT oligomerization. Lysenin monomers bind specifically to sphingomyelin via the receptor binding domain.[12] The final lysenin oligomer is constituted by nine monomers without quantified deviations.[13] When lysenin monomers bind to sphingomyelin-enriched membrane regions, they provide a stable platform with a high lateral mobility, hence favouring the oligomerization.[14] As with most PFTs, lysenin oligomerization occurs in a two-step process, as was recently imaged.
The process begins with monomers being adsorbed into the membrane by specific interactions, resulting in an increased concentration of monomers. This increase is promoted by the small area where the membrane receptor accumulates owing to the fact that the majority of PFT membrane receptors are associated with lipid rafts.[15] Another side effect, aside from the increase of monomer concentration, is the monomer-monomer interaction. This interaction increases lysenin oligomerization. After a critical threshold concentration is reached, several oligomers are formed simultaneously, although sometimes these are incomplete.[16] In contrast to PFTs of the cholesterol-dependent cytolysin family,[17] the transition from incomplete lysenin oligomers to complete oligomers has not been observed.
A complete oligomerization results in the so-called prepore state, a structure on the membrane. Determining the prepore's structure by X-ray or Cryo-EM is a challenging process that so far has not produced any results. The only available information about the prepore structure was provided by Atomic Force Microscopy (AFM). The measured prepore height was 90 Å; and the width 118 Å, with an inner pore of 50 Å.[16] A model of the prepore was built aligning the monomer structure (PDB: 3ZXD) with the pore structure (PDB: 5GAQ) by their receptor-binding domains (residues 160 to 297). A recent study in aerolysin suggests that the currently accepted model for the lysenin prepore should be revisited, according to the new available data on the aerolysin insertion.
A conformational change transforms the PFM into the transmembrane β-barrel, leading to the pore state.[18] The trigger mechanism for the prepore-to-pore transition in lysenin depends on three glutamic acid residues (E92, E94 and E97), and is activated by a decrease in pH,[19] from physiological conditions to the acidic conditions reached after endocytosis. These three glutamic acids are located in an α-helix that forms part of the PFM, and glutamic acids are found in aerolysin family members in its PFMs. Such a conformational change produces a decrease in the oligomer height of 2.5 nm according to AFM measurements.[16] The main dimensions, using lysenin pore X-ray structure, are height 97 Å, width 115 Å and the inner pore of 30 Å.[18] However, complete oligomerization into the nonamer is not a requisite for the insertion, since incomplete oligomers in the pore state can be found.[16] The prepore to pore transition can be blocked in crowded conditions, a mechanism that could be general to all β-PFTs. The first hint of crowding effect on prepore to pore transition was given by congestion effects in electrophysiology experiments.[20] High-Speed AFM studies incubating lysenin on sphingomyelin/cholesterol membranes has shown that under crowded conditions the prepore to pore transition gets blocked by steric interactions.[21]
Insertion consequences
The ultimate consequences of lysenin pore formation are not well documented; however, it is through to induce apoptosis via three possible hypotheses:
- Breaking the sphingomyelin asymmetry between the two leaflets of the lipid bilayer by punching holes in the membrane[22] and inducing lipid flip-flop (reorientation of a lipid from one leaflet of a membrane bilayer to the other).[23]
- Increasing the calcium concentration in the cytoplasm.[24]
- Decreasing the potassium concentration in the cytoplasm.[25]
Biological role
The biological role of lysenin remains unknown. It has been suggested that lysenin may play a role as a defence mechanism against attackers such as bacteria, fungi or small invertebrates.[26] However, lysenin's activity is dependent upon binding to sphingomyelin, which is not present in the membranes of bacteria, fungi or most invertebrates. Rather, sphingomyelin is mainly present in the plasma membrane of chordates.[27] Another hypothesis is that the earthworm, which is able to expel coelomic fluid under stress,[28][29] generates an avoidance behaviour to its vertebrate predators (such as birds, hedgehogs or moles).[30] If that is the case, the expelled lysenin might be more effective if the coelomic fluid reaches the eye, where the concentration of sphingomyelin is ten times higher than in other body organs.[31] A complementary hypothesis is that the pungent smell of the coelomic fluid - giving the earthworm its specific epithet foetida - is an anti-predator adaptation. However, it remains unknown whether lysenin contributes to avoidance of Eisenia by predators.[32]
Applications
Lysenin's conductive properties have been studied for years.[33] Like most pore-forming toxins, lysenin forms a non-specific channel that is permeable to ions, small molecules, and small peptides.[34] There have also been over three decades of studies into finding suitable pores for converting into nanopore sequencing systems that can have their conductive properties tuned by point mutation.[35] Owing to its binding affinity for sphingomyelin, lysenin (or just the receptor binding domain) has been used as a fluorescence marker to detect the sphingomyelin domain in membranes.[36]
References
- ^ Yilmaz, N.; Yamaji-Hasegawa, A.; Hullin-Matsuda, F.; Kobayashi, T. (2018). "Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin". Seminars in Cell & Developmental Biology. 73: 188–198. doi:10.1016/j.semcdb.2017.07.036.
- ^ Sekizawa, Y.; Hagiwara, K.; Nakajima, T.; Kobayashi, H. (1996). "A Novel Protein, Lysenin, that Causes Contraction of the Isolate Rat Aorta: Its Purification from the Coelomic Fluid of the Earthworm, Eisenia foetida". Biomedical Research. 17 (3): 197–203. doi:10.2220/biomedres.17.197.
- ^ a b De Colibus, L.; Sonnen, A. F.-P.; Morris, K. J.; Siebert, C. A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; Gilbert, R. J. C. (2012). "Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition". Structure. 20 (9): 1498–1507. doi:10.1016/j.str.2012.06.011.
- ^ Bokori-Brown, M.; Martin, T. G.; Naylor, C. E.; Basak, A. K.; Titball, R. W.; Savva, C. G. (2016). "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". Nature Communications. 7 (1). doi:10.1038/ncomms11293.
- ^ a b Ishitsuka, R.; Kobayashi, T. (2007). "Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin". Biochemistry. 46 (6): 1495–1502. doi:10.1021/bi061290k.
- ^ Simons, K.; Gerl, M. J. (2010). "Revitalizing membrane rafts: new tools and insights". Nature Reviews Molecular Cell Biology. 11 (10): 688–699. doi:10.1038/nrm2977.
- ^ Ros, U.; García-Sáez, A. J. (2015). "More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes". The Journal of Membrane Biology. 248 (3): 545–561. doi:10.1007/s00232-015-9820-y.
- ^ Yilmaz, N.; Kobayashi, T. (2015). "Visualization of Lipid Membrane Reorganization Induced by a Pore-Forming Toxin Using High-Speed Atomic Force Microscopy". ACS Nano. 9 (8): 7960–7967. doi:10.1021/acsnano.5b01041.
- ^ Bokori-Brown, M.; Martin, T. G.; Naylor, C. E.; Basak, A. K.; Titball, R. W.; Savva, C. G. (2016). "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". Nature Communications. 7 (1). doi:10.1038/ncomms11293.
- ^ Quinn, P. J. (2013). "Structure of Sphingomyelin Bilayers and Complexes with Cholesterol Forming Membrane Rafts". Langmuir. 29 (30): 9447–9456. doi:10.1021/la4018129.
- ^ Guigas, G.; Weiss, M. (2016). "Effects of protein crowding on membrane systems". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1858 (10): 2441–2450. doi:10.1016/j.bbamem.2015.12.021.
- ^ De Colibus, L.; Sonnen, A. F.-P.; Morris, K. J.; Siebert, C. A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; Gilbert, R. J. C. (2012). "Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins And Its Mode of Sphingomyelin Recognition". Structure. 20 (9): 1498–1507. doi:10.1016/j.str.2012.06.011.
- ^ Munguira, I.; Casuso, I.; Takahashi, H.; Rico, F.; Miyagi, A.; Chami, M.; Scheuring, S. (2016). "Glasslike Membrane Protein Diffusion in a Crowded Membrane". ACS Nano. 10 (2): 2584–2590. doi:10.1021/acsnano.5b07595.
- ^ Ishitsuka, R.; Kobayashi, T. (2007). "Cholesterol and Lipid/Protein Ratio Control the Oligomerization of a Sphingomyelin-Specific Toxin, Lysenin". Biochemistry. 46 (6): 1495–1502. doi:10.1021/bi061290k.
- ^ Lafont, F.; Van Der Goot, F. G. (2005). "Bacterial invasion via lipid rafts". Cellular Microbiology. 7 (5): 613–620. doi:10.1111/j.1462-5822.2005.00515.x.
- ^ a b c d Yilmaz, N.; Yamada, T.; Greimel, P.; Uchihashi, T.; Ando, T.; Kobayashi, T. (2013). "Real-Time Visualization of Assembling of a Sphingomyelin-Specific Toxin on Planar Lipid Membranes". Biophysical Journal. 105 (6): 1397–1405. doi:10.1016/j.bpj.2013.07.052.
- ^ Mulvihill, E.; van Pee, K.; Mari, S. A.; Müller, D. J.; Yildiz, Ö. (2015). "Directly Observing the Lipid-Dependent Self-Assembly and Pore-Forming Mechanism of the Cytolytic Toxin Listeriolysin O". Nano Letters. 15 (10): 6965–6973. doi:10.1021/acs.nanolett.5b02963.
- ^ a b Bokori-Brown, M.; Martin, T. G.; Naylor, C. E.; Basak, A. K.; Titball, R. W.; Savva, C. G. (2016). "Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein". Nature Communications. 7 (1). doi:10.1038/ncomms11293.
- ^ Munguira, I. L. B.; Takahashi, H.; Casuso, I.; Scheuring, S. (2017). "Lysenin Toxin Membrane Insertion Is pH-Dependent but Independent of Neighboring Lysenins". Biophysical Journal. 113 (9): 2029–2036. doi:10.1016/j.bpj.2017.08.056.
- ^ Krueger, E.; Bryant, S.; Shrestha, N.; Clark, T.; Hanna, C.; Pink, D.; Fologea, D. (2015). "Intramembrane congestion effects on lysenin channel voltage-induced gating". European Biophysics Journal. 45 (2): 187–194. doi:10.1007/s00249-015-1104-z.
- ^ Munguira, I. L. B. (2017). Effect of Crowdedness in the Life Cycle of Lysenin Studied by High-Speed Atomic Force Microscopy (PhD). Aix-Marseille Universite.
- ^ Green, D. R. (2000). "Apoptosis and Sphingomyelin Hydrolysis". The Journal of Cell Biology. 150 (1): F5–F8. doi:10.1083/jcb.150.1.F5.
- ^ Ros, U.; García-Sáez, A. J. (2015). "More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes". The Journal of Membrane Biology. 248 (3): 545–561. doi:10.1007/s00232-015-9820-y.
- ^ Orrenius, S.; Zhivotovsky, B.; Nicotera, P. (2003). "Regulation of cell death: the calcium–apoptosis link". Nature Reviews Molecular Cell Biology. 4 (7): 552–565. doi:10.1038/nrm1150.
- ^ Yu, S. P. (2003). "Regulation and critical role of potassium homeostasis in apoptosis". Progress in Neurobiology. 70 (4): 363–386. doi:10.1016/s0301-0082(03)00090-x.
- ^ Ballarin, L.; Cammarata, M. (2016). Lessons in immunity: from single-cell organisms to mammals. Academic Press. ISBN 9780128032527.
- ^ Kobayashi, H.; Sekizawa, Y.; Aizu, M.; Umeda, M. (2000). "Lethal and non-lethal responses of spermatozoa from a wide variety of vertebrates and invertebrates to lysenin, a protein from the coelomic fluid of the earthworm Eisenia foetida". Journal of Experimental Zoology. 286 (5): 538–549. doi:10.1002/(sici)1097-010x(20000401)286:5<538::aid-jez12>3.0.co;2-w.
- ^ Sukumwang, N.; Umezawa, K. (2013). "Earthworm-Derived Pore-Forming Toxin Lysenin and Screening of Its Inhibitors". Toxins. 5 (8): 1392–1401. doi:10.3390/toxins5081392.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kobayashi, H.; Ohta, N.; Umeda, M. (2004). "Biology of lysenin, a protein in the coelomic fluid of the earthworm Eisenia foetida". International Review of Cytology. 236: 45–99. doi:10.1016/S0074-7696(04)36002-X. PMID 15261736.
- ^ Swiderska, B.; Kedracka-Krok, S.; Panz, T.; Morgan, A. J.; Falniowski, A.; Grzmil, P.; Plytycz, B. (2017). "Lysenin family proteins in earthworm coelomocytes – Comparative approach". Developmental & Comparative Immunology. 67: 404–412. doi:10.1016/j.dci.2016.08.011.
- ^ Berman, E. R. (1991). Biochemistry of the Eye. Springer. doi:10.1007/978-1-4757-9441-0. ISBN 978-1-4757-9441-0.
- ^ Edwards, C. A.; Bohlen, P. J. (1996). Biology and Ecology of Earthworms. Springer Science & Business Media. ISBN 978-0-412-56160-3.
- ^ Bryant, S.; Clark, T.; Thomas, C.; Ware, K.; Bogard, A.; Calzacorta, C.; Prather, D.; Fologea, D. (2018). "Insights into the Voltage Regulation Mechanism of the Pore-Forming Toxin Lysenin". Toxins. 10 (8): 334. doi:10.3390/toxins10080334.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shrestha, N.; Bryant, S. L.; Thomas, C.; Richtsmeier, D.; Pu, X.; Tinker, J.; Fologea, D. (2017). "Stochastic sensing of Angiotensin II with lysenin channels". Scientific Reports. 7 (1). doi:10.1038/s41598-017-02438-0.
- ^ Deamer, D.; Akeson, M.; Branton, D. (2016). "Three decades of nanopore sequencing". Nature Biotechnology. 34 (5): 518–524. doi:10.1038/nbt.3423.
- ^ Ishitsuka, R.; Kobayashi, T. (2004). "Lysenin: A new tool for investigating membrane lipid organization". Anatomical Science International. 79 (4): 184–190. doi:10.1111/j.1447-073x.2004.00086.x.
