Whiting event: Difference between revisions
Article deOrphaned! |
Updated lead section and added "Characteristics," "Potential causes," and "Relevance" sections |
||
| Line 1: | Line 1: | ||
[[File:Lake Ontario Whiting NASA Satellite Image.jpg|thumb|An aerial view of a whiting event precipitation cloud in Lake Ontario.]] |
[[File:Lake Ontario Whiting NASA Satellite Image.jpg|thumb|An aerial view of a whiting event precipitation cloud in Lake Ontario.]] |
||
A '''whiting event''' is a phenomenon that occurs when a [[calcium carbonate]] precipitate |
A '''whiting event''' is a phenomenon that occurs when a cloud of [[calcium carbonate]] precipitate forms in [[Body of water|water bodies]], typically during summer months, as a result of [[Photosynthesis|photosynthetic]] microbiological activity or [[Abiotic component|abiotic]] factors<ref name=":0" /><ref name=":1">{{Cite journal|last=Larson|first=Erik B.|last2=Mylroie|first2=John E.|date=2014|title=A review of whiting formation in the Bahamas and new models|url=http://dx.doi.org/10.1007/s13146-014-0212-7|journal=Carbonates and Evaporites|volume=29|issue=4|pages=337–347|doi=10.1007/s13146-014-0212-7|issn=0891-2556|via=}}</ref><ref name=":4" />. The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters<ref name=":4">{{Cite journal|last=Sondi|first=Ivan|last2=Juračić|first2=Mladen|date=2010|title=Whiting events and the formation of aragonite in Mediterranean Karstic Marine Lakes: new evidence on its biologically induced inorganic origin|url=https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3091.2009.01090.x|journal=Sedimentology|language=en|volume=57|issue=1|pages=85–95|doi=10.1111/j.1365-3091.2009.01090.x|issn=1365-3091}}</ref>. They can also occur in both marine and freshwater environments<ref name=":5">{{Cite journal|last=Long|first=Jacqueline S.|last2=Hu|first2=Chuanmin|last3=Robbins|first3=Lisa L.|last4=Byrne|first4=Robert H.|last5=Paul|first5=John H.|last6=Wolny|first6=Jennifer L.|date=2017|title=Optical and biochemical properties of a southwest Florida whiting event|url=http://dx.doi.org/10.1016/j.ecss.2017.07.017|journal=Estuarine, Coastal and Shelf Science|volume=196|pages=258–268|doi=10.1016/j.ecss.2017.07.017|issn=0272-7714|via=}}</ref>. The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom [[sediment]] re-suspension or by increased activity of certain microscopic life such as [[phytoplankton]]<ref>{{cite journal |doi=10.4319/lo.1997.42.1.0133 |title=Whiting events: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton |date=1997 |last1=Thompson |first1=Joel B. |last2=Schultze-Lam |first2=Susanne |last3=Beveridge |first3=Terrance J. |last4=Des Marais |first4=David J. |journal=Limnology and Oceanography |volume=42 |pages=133–41 |pmid=11541205 |issue=1}}</ref><ref>{{cite web|url=http://earthobservatory.nasa.gov/IOTD/view.php?id=1768|title=Whiting in Lake Michigan|publisher=NASA Earth Observatory}}</ref><ref name=":0">{{cite web|url=http://earthobservatory.nasa.gov/IOTD/view.php?id=81952|title=Whiting Event, Lake Ontario|publisher=NASA Earth Observatory}}</ref>. |
||
<br /> |
|||
== Characteristics == |
|||
Whiting event clouds consist of calcium carbonate [[Polymorphism (materials science)|polymorphs]]; [[aragonite]] tends to be the dominant precipitate, but some studies in [[Trophic state index|oligotrophic]] and [[Trophic state index|mesotrophic]] lakes favor [[calcite]]<ref name=":4" /><ref name=":2">{{Cite journal|last=Dittrich|first=Maria|last2=Obst|first2=Martin|date=2004|title=Are Picoplankton Responsible for Calcite Precipitation in Lakes?|url=http://www.bioone.org/doi/abs/10.1579/0044-7447-33.8.559|journal=AMBIO: A Journal of the Human Environment|language=en|volume=33|issue=8|pages=559–564|doi=10.1579/0044-7447-33.8.559|issn=0044-7447|via=}}</ref>.Whiting events have been observed in tropical as well as temperate waters and can potentially cover hundreds of meters<ref name=":4" />. They tend to occur more often in summer months, as warmer waters promote calcium carbonate precipitation, and in [[Hard water|hard waters]]<ref name=":4" /><ref name=":3">{{Cite journal|last=Effler|first=Steven W.|last2=Perkins|first2=Mary Gail|last3=Greer|first3=Harry|last4=Johnson|first4=David L.|date=1987|title=Effect of "whiting" on optical properties and turbidity in Owasco Lake, New York|url=http://doi.wiley.com/10.1111/j.1752-1688.1987.tb00796.x|journal=Journal of the American Water Resources Association|language=en|volume=23|issue=2|pages=189–196|doi=10.1111/j.1752-1688.1987.tb00796.x|issn=1093-474X|via=}}</ref>. Whitings are typically characterized by cloudy, white patches of water, but they have been observed to be tanner in hue in very shallow waters (less than 5m deep)<ref name=":1" />. These shallow water whiting events also tend to last less than a day in comparison to deeper water events that can last for several days up to several weeks<ref name=":1" />. Regardless of the event's lifespan, the clouds it produces increase [[turbidity]] and hamper light penetration<ref name=":3" />. |
|||
== Potential causes == |
|||
Some debate exists surrounding the exact cause of whiting events. And although much research exists on the subject, there is still no definitive consensus on the chemical mechanisms behind it. The three most common suggested causes for the phenomenon are: microbiological processes, re-suspension of marine or bottom sediments, and spontaneous direct precipitation from water<ref name=":4" /><ref name=":1" />. Of these three, the last has been ruled unlikely due to the unfavorable [[Reaction Kinetics|reaction kinetics]] of spontaneous calcium carbonate precipitation<ref name=":1" />. |
|||
=== Microbiological activity === |
|||
Substantial findings indicate [[Photosynthesis|photosynthetic]] [[picoplankton]], pico[[cyanobacteria]], and [[phytoplankton]] activity creates favorable conditions for carbonate precipitation<ref name=":4" /><ref name=":1" /><ref name=":2" />. This link arises as a result of [http://www.genomenewsnetwork.org/articles/02_02/bloom_art.shtml planktonic blooms] being observed coinciding with the events<ref name=":1" /><ref name=":2" />. Subsequently, via photosynthesis, these organisms uptake [[Inorganic carbon compound|inorganic carbon]], raise water pH, and alter water [[alkalinity]], which promotes calcium carbonate precipitation<ref name=":1" /><ref name=":2" />. Furthermore, cases exist in which the type of calcium carbonate found in the whiting cloud matches the type found on local cyanobacteria membranes<ref name=":5" />. It's hypothesized that the [[Extracellular polymeric substance|extracellular polymeric substances (EPS)]] these microorganisms produce can act as [[Seed crystal|seed crystals]] that provide a start for the precipitation process<ref name=":1" /><ref name=":2" />. Current research on the specifics of these EPS and the exact physiological mechanisms of carbon uptake, however, are limited<ref name=":1" /><ref name=":2" />. |
|||
=== Sediment re-suspension === |
|||
In shallower waters, evidence supports that activity of local fisherman and marine life such as fish and certain shark species can disturb bottom sediments containing calcium carbonate particles and lead to their suspension<ref name=":1" />. In addition, as microorganisms impact water chemistry in observable ways and require certain nutrient levels to thrive, whiting events found occurring in nutrient-poor waters where no significant alkalinity difference exists between whiting and non-whiting waters support the idea of sediment re-suspension as a primary cause<ref>{{Cite journal|last=Morse|first=John W.|last2=Gledhill|first2=Dwight K.|last3=Millero|first3=Frank J.|date=2003|title=Caco3 precipitation kinetics in waters from the great Bahama bank:|url=https://linkinghub.elsevier.com/retrieve/pii/S0016703703001030|journal=Geochimica et Cosmochimica Acta|language=en|volume=67|issue=15|pages=2819–2826|doi=10.1016/S0016-7037(03)00103-0|via=}}</ref>. |
|||
== Relevance == |
|||
Whiting events have a unique effect on the waters around them. The fact that calcium carbonate clouds increase turbidity and light reflectance holds implications for organisms and processes that depend on light<ref name=":5" />. In addition, whiting events can function as a transport mechanism for organic carbon to the [[benthic zone]], which is relevant to [[nutrient cycling]]<ref>{{Cite journal|last=Hodell|first=David A.|last2=Schelske|first2=Claire L.|date=1998|title=Production, sedimentation, and isotopic composition of organic matter in Lake Ontario|url=http://dx.doi.org/10.4319/lo.1998.43.2.0200|journal=Limnology and Oceanography|volume=43|issue=2|pages=200–214|doi=10.4319/lo.1998.43.2.0200|issn=0024-3590|via=}}</ref>. The cyanobacteria abundant clouds also hold the potential to act as a means to study the microorganism's role in carbon cycling (especially in relation to climate change) and possible role as petroleum [[Source rock|source rocks]]<ref>{{Cite book|title=AAPG Studies in Geology|last=Yates|first=K.K|last2=Robbins|first2=L.L.|publisher=American Association of Petroleum Geologists|year=2001|isbn=|location=Tulsa, Ok|pages=267-283|chapter=Microbial Lime-Mud Production and Its Relation to Climate Change}}</ref><ref>{{Cite journal|last=Shinn|first=Eugene A.|last2=St.C. Kendall|first2=Christopher G.|date=2011-12-01|editor-last=Day-Stirrat|editor-first=Ruarri|editor2-last=Janson|editor2-first=Xavier|editor3-last=Wright|editor3-first=Wayne|title=Back to the Future|url=https://www.sepm.org/files/94article.555sjklz3vi6izko.pdf|journal=The Sedimentary Record|volume=9|issue=4|pages=4–9|doi=10.2110/sedred.2011.4.4}}</ref>. |
|||
==References== |
==References== |
||
Revision as of 01:49, 22 November 2019
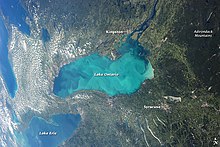
A whiting event is a phenomenon that occurs when a cloud of calcium carbonate precipitate forms in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or abiotic factors[1][2][3]. The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters[3]. They can also occur in both marine and freshwater environments[4]. The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton[5][6][1].
Characteristics
Whiting event clouds consist of calcium carbonate polymorphs; aragonite tends to be the dominant precipitate, but some studies in oligotrophic and mesotrophic lakes favor calcite[3][7].Whiting events have been observed in tropical as well as temperate waters and can potentially cover hundreds of meters[3]. They tend to occur more often in summer months, as warmer waters promote calcium carbonate precipitation, and in hard waters[3][8]. Whitings are typically characterized by cloudy, white patches of water, but they have been observed to be tanner in hue in very shallow waters (less than 5m deep)[2]. These shallow water whiting events also tend to last less than a day in comparison to deeper water events that can last for several days up to several weeks[2]. Regardless of the event's lifespan, the clouds it produces increase turbidity and hamper light penetration[8].
Potential causes
Some debate exists surrounding the exact cause of whiting events. And although much research exists on the subject, there is still no definitive consensus on the chemical mechanisms behind it. The three most common suggested causes for the phenomenon are: microbiological processes, re-suspension of marine or bottom sediments, and spontaneous direct precipitation from water[3][2]. Of these three, the last has been ruled unlikely due to the unfavorable reaction kinetics of spontaneous calcium carbonate precipitation[2].
Microbiological activity
Substantial findings indicate photosynthetic picoplankton, picocyanobacteria, and phytoplankton activity creates favorable conditions for carbonate precipitation[3][2][7]. This link arises as a result of planktonic blooms being observed coinciding with the events[2][7]. Subsequently, via photosynthesis, these organisms uptake inorganic carbon, raise water pH, and alter water alkalinity, which promotes calcium carbonate precipitation[2][7]. Furthermore, cases exist in which the type of calcium carbonate found in the whiting cloud matches the type found on local cyanobacteria membranes[4]. It's hypothesized that the extracellular polymeric substances (EPS) these microorganisms produce can act as seed crystals that provide a start for the precipitation process[2][7]. Current research on the specifics of these EPS and the exact physiological mechanisms of carbon uptake, however, are limited[2][7].
Sediment re-suspension
In shallower waters, evidence supports that activity of local fisherman and marine life such as fish and certain shark species can disturb bottom sediments containing calcium carbonate particles and lead to their suspension[2]. In addition, as microorganisms impact water chemistry in observable ways and require certain nutrient levels to thrive, whiting events found occurring in nutrient-poor waters where no significant alkalinity difference exists between whiting and non-whiting waters support the idea of sediment re-suspension as a primary cause[9].
Relevance
Whiting events have a unique effect on the waters around them. The fact that calcium carbonate clouds increase turbidity and light reflectance holds implications for organisms and processes that depend on light[4]. In addition, whiting events can function as a transport mechanism for organic carbon to the benthic zone, which is relevant to nutrient cycling[10]. The cyanobacteria abundant clouds also hold the potential to act as a means to study the microorganism's role in carbon cycling (especially in relation to climate change) and possible role as petroleum source rocks[11][12].
References
- ^ a b "Whiting Event, Lake Ontario". NASA Earth Observatory.
- ^ a b c d e f g h i j k Larson, Erik B.; Mylroie, John E. (2014). "A review of whiting formation in the Bahamas and new models". Carbonates and Evaporites. 29 (4): 337–347. doi:10.1007/s13146-014-0212-7. ISSN 0891-2556.
- ^ a b c d e f g Sondi, Ivan; Juračić, Mladen (2010). "Whiting events and the formation of aragonite in Mediterranean Karstic Marine Lakes: new evidence on its biologically induced inorganic origin". Sedimentology. 57 (1): 85–95. doi:10.1111/j.1365-3091.2009.01090.x. ISSN 1365-3091.
- ^ a b c Long, Jacqueline S.; Hu, Chuanmin; Robbins, Lisa L.; Byrne, Robert H.; Paul, John H.; Wolny, Jennifer L. (2017). "Optical and biochemical properties of a southwest Florida whiting event". Estuarine, Coastal and Shelf Science. 196: 258–268. doi:10.1016/j.ecss.2017.07.017. ISSN 0272-7714.
- ^ Thompson, Joel B.; Schultze-Lam, Susanne; Beveridge, Terrance J.; Des Marais, David J. (1997). "Whiting events: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton". Limnology and Oceanography. 42 (1): 133–41. doi:10.4319/lo.1997.42.1.0133. PMID 11541205.
- ^ "Whiting in Lake Michigan". NASA Earth Observatory.
- ^ a b c d e f Dittrich, Maria; Obst, Martin (2004). "Are Picoplankton Responsible for Calcite Precipitation in Lakes?". AMBIO: A Journal of the Human Environment. 33 (8): 559–564. doi:10.1579/0044-7447-33.8.559. ISSN 0044-7447.
- ^ a b Effler, Steven W.; Perkins, Mary Gail; Greer, Harry; Johnson, David L. (1987). "Effect of "whiting" on optical properties and turbidity in Owasco Lake, New York". Journal of the American Water Resources Association. 23 (2): 189–196. doi:10.1111/j.1752-1688.1987.tb00796.x. ISSN 1093-474X.
- ^ Morse, John W.; Gledhill, Dwight K.; Millero, Frank J. (2003). "Caco3 precipitation kinetics in waters from the great Bahama bank:". Geochimica et Cosmochimica Acta. 67 (15): 2819–2826. doi:10.1016/S0016-7037(03)00103-0.
- ^ Hodell, David A.; Schelske, Claire L. (1998). "Production, sedimentation, and isotopic composition of organic matter in Lake Ontario". Limnology and Oceanography. 43 (2): 200–214. doi:10.4319/lo.1998.43.2.0200. ISSN 0024-3590.
- ^ Yates, K.K; Robbins, L.L. (2001). "Microbial Lime-Mud Production and Its Relation to Climate Change". AAPG Studies in Geology. Tulsa, Ok: American Association of Petroleum Geologists. pp. 267–283.
- ^ Shinn, Eugene A.; St.C. Kendall, Christopher G. (2011-12-01). Day-Stirrat, Ruarri; Janson, Xavier; Wright, Wayne (eds.). "Back to the Future" (PDF). The Sedimentary Record. 9 (4): 4–9. doi:10.2110/sedred.2011.4.4.
Further reading
- Thompson, Joel B.; et al. (1997). "Whiting Event: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton" (PDF). American Society of Limnology and Oceanography. pp. 133–141.
- Effler, Steven W.; Perkins, Mary Gail; Greer, Harry; Johnson, David L. (1987). "Effect of 'whiting' on Optical Properties and Turbidity in Owasco Lake, New York". Journal of the American Water Resources Association. 23 (2): 189–96. doi:10.1111/j.1752-1688.1987.tb00796.x.
- "Whiting Events(Calcium Carbonate Precipitate)A naturally occurring phenomena" (PDF).
