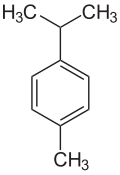
P-Cymene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-Methyl-4-(1-methylethyl)benzene
| |||
| Other names
4-Isopropyltoluene; Paracymene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.542 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H14 | |||
| Molar mass | 134.21 g/mol | ||
| Appearance | Colourless liquid | ||
| Density | 0.857 g/cm3 | ||
| Melting point | -68°C | ||
| Boiling point | 177°C | ||
| 23.4 mg/L | |||
| Hazards | |||
| Flash point | 47°C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cymene, or p-cymene, is a naturally occurring aromatic organic compound. It is classified as a hydrocarbon related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. It is insoluble in water, but miscible with ethanol and ether.
Cymene is a constituent of a number of essential oils, most commonly the oil of cumin and thyme.
There are two less common geometric isomers. o-Cymene, in which the alkyl groups are ortho-substituted, and m-cymene, in which they are meta-substituted. p-Cymene is the only natural isomer.
Cymene is a common ligand for ruthenium. The parent compound is [(η6-cymene)RuCl2]2. This half-sandwich compound is prepared by the reaction of ruthenium trichloride with the terpene α-phellandrene. The osmium complex is also known.[1]
Significant amounts of cymene are formed in sulfite pulping process from the wood terpenes.
References
- ^ Bennett, M. A.; Huang, T. N.; Matheson, T. W. and Smith, A. K. (1982). "(h6-Hexamethylbenzene)ruthenium complexes". Inorganic Syntheses. 21: 74–8. doi:10.1002/9780470132524.ch16.
{{cite journal}}: CS1 maint: multiple names: authors list (link)