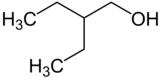
2-Ethyl-1-butanol
| |
| Names | |
|---|---|
| IUPAC name
2-Ethylbutan-1-ol[2]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1731254 | |
| ChemSpider | |
| ECHA InfoCard | 100.002.384 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2275 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.177 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 830 mg mL−1 |
| Melting point | −114.40 °C; −173.92 °F; 158.75 K |
| Boiling point | 145 °C; 293 °F; 418 K |
| 10 g L−1 | |
| Vapor pressure | 206 Pa |
Refractive index (nD)
|
1.422 |
| Thermochemistry | |
Heat capacity (C)
|
246.65 J K−1 mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312 | |
| P280 | |
| Flash point | 58 °C (136 °F; 331 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1.85 g kg−1 (oral, rat) |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Ethyl-1-butanol (IUPAC name) is an organic chemical compound. It can be used to facilitate the separation of ethanol from water, which form an azeotrope that otherwise limits the maximum ethanol concentration.[3]
Reactions
2-Ethyl-1-butanol is manufactured industrially by the aldol condensation of acetaldehyde and butyraldehyde.[4]
References
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–262, 8–106, 15–20, ISBN 0-8493-0594-2
- ^ "2-ethyl-1-butanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 28 January 2012.
- ^ Roddy, James W. (1981). "Distribution of ethanol-water mixtures to organic liquids". Ind. Eng. Chem. Proc. Des. Dev. 20 (1). American Chemical Society: 104–108. doi:10.1021/i200012a016.
- ^ McKetta, John J.; Cunningham, William Aaron (1994), Encyclopedia of Chemical Processing and Design, vol. 47, Boca Raton, FL: CRC Press, p. 117, ISBN 978-0-8247-2451-1, retrieved 2010-01-25
