Aggressive lymphoma
| Aggressive Lymphoma | |
|---|---|
 | |
| Diffuse Large B-cell Lymphoma | |
| Specialty | Hematology and oncology |
Aggressive lymphoma, also known as high-grade lymphoma, is a group of fast growing non-Hodgkin lymphoma.[1]
Some common symptoms for aggressive lymphoma are weight loss, night sweats, nausea and recurrent fevers.[2] Since these tumors are fast to grow and spread, immediate intervention is required after diagnosis.[citation needed]
There are several subtypes of aggressive lymphoma. These include AIDS-associated lymphoma, angioimmunoblastic lymphoma, Burkitt lymphoma, central nervous system (CNS) lymphoma, diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL) and peripheral T-cell lymphoma.[1] Diffuse large B-cell lymphoma is the most common subtype as well as the most common type of non-Hodgkin lymphoma.[3]
Aggressive lymphoma accounts for approximately 60 percent of all non-Hodgkin cases in the United States.[1]
Types
The most common types of high grade non-Hodgkin lymphoma are:[4]
Diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL) is a type of high-grade NHL which is the most common type of NHL worldwide at affecting 30–40 percent of all NHL cases.[5] It is more prevalent in elderly patients with a median age of 70 years. Patients most often present with a rapidly growing tumor mass in single or multiple, nodal or extranodal sites. The most commonly diagnosed versions of DLBCL are germinal center B-cell like (GCB) and activated B-cell like (ABC) subtypes.[6]
Immunohistochemical and flow cytometry analysis is also necessary for the diagnosis of this disease. The DLBCL cells have found to express B-cell antigens such as CD19, CD20, and CD22 as well as the transcription factors PAX5, BOB.1, and OCT2. CD20 is especially relevant in diagnostics as well as therapeutics because it is the target of the Rituximab humanized monoclonal antibody that is used in the most common treatment strategy.[6] In addition to this, the majority of cells also express surface or cytoplasmic immunoglobulins, specifically IgM, IgG and IgA. While these analyses allow for diagnosis by displaying proof of B-cell lineage, they can also be used in treatment strategy and can give insight into prognosis of the specific case being considered.[citation needed]
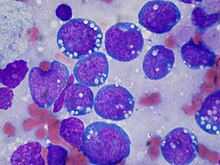
Burkitt lymphoma
Burkitt lymphoma is named after the British surgeon Denis Burkitt. Burkitt identified this disease in 1956 in children in Africa.[7] In Africa, Burkitt lymphoma is common in children also infected by malaria and Epstein–Barr virus.[citation needed]
Outside of Africa, Burkitt lymphoma is less common. In the U.S., about 1,200 people are diagnosed each year, and the disease disproportionately affects those younger than the age of 40.[7] Burkitt lymphoma is especially likely to develop in people infected with HIV, the virus that causes AIDS. Before highly active antiretroviral therapy (HAART) became a widespread treatment for HIV/AIDS, the incidence of Burkitt lymphoma was estimated to be 1,000 times higher in HIV-positive people than in the general population.[7]
Peripheral T-cell lymphoma
Peripheral T-cell lymphomas are less common accounting for approximately 7% of all non-Hodgkin lymphoma cases.[8] It represents a diverse group of diseases that lacks clear definition. These diseases may involve extranodal sites such as the liver, bone, marrow, intestinal tract and the skin.[8] Due to its difficulties in classification, diagnosis and treatment is often regarded as "orphan diseases".[citation needed]
Rarer types of high grade non-Hodgkin lymphoma
- AIDS-associated lymphoma
- Angioimmunoblastic lymphoma
- Central nervous system (CNS) lymphoma
- Mantle cell lymphoma (MCL)
Risk factors
Some of the risk factors that increase the risk factor of aggressive lymphoma include:[9]
- Immunosuppressive medication used for organ transplant patients or autoimmune diseases.
- Infection with certain viruses and bacteria such as HIV and Epstein–Barr virus (EBV).
- Exposure to chemicals such as insecticides and pesticides.
- Older age.
Diagnosis
The symptoms of aggressive lymphoma are similar to that of low-grade lymphomas. Patients may come in with painless swollen lymph nodes in their neck, armpits or groin. They can also describe symptoms such as abdominal pain, chest pain, persistent fatigue, fever, night sweats and unexplained weight loss depending on the stage of their tumor and its location.[9]
Since the presentation of aggressive lymphoma are similar to that of other types of non-Hodgkin lymphoma it is important analyze the tumors morphologically and run immunohistochemical tests to ensure correct diagnosis. For aggressive lymphoma, swift and specific treatment dependent upon correct diagnosis can be the difference between life and death for the patient.[citation needed]
Treatment
For limited high grade lymphoma the typical treatment is chemotherapy and a targeted drug, followed by radiotherapy. While advanced high grade aggressive lymphoma patients receive similar treatments, the duration is extended.[citation needed]
For advanced diffuse large B-cell lymphoma the standard therapy for patients with DLBCL is the combination use of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).[3] While this commonly used regimen results in 60–70 percent of patients with DLBCL to be cured of disease there is also a group of patients that relapse and do not respond well to R-CHOP. For these patients the current treatment strategies are insufficient in treating their cancer. In addition to this, alternative treatments continue to be research due to the highly toxic nature of R-CHOP.[3]
In contrast to mature B-cell neoplasms, therapeutic advances in peripheral T-cell lymphoma have generally lagged behind, particularly with regard to the introduction of immune therapies, which have impacted significantly and favorably on the prognosis of aggressive B cell lymphomas such as diffuse large B-cell lymphoma. As such, it remains a heterogeneous entity with a generally unfavorable prognosis.[10]
Prognosis
While there is no way of definitively predicting the outcome of an individual's diagnosis of aggressive lymphoma, there are ways of best guessing the prognosis of the patient. These prognostic factors can be divided into three categories. The first category depends on the patient and can be their age and overall health. The second category is dependent upon the tumor and includes the stage, tumor burden, and extranodal involvement. Finally, the last category is dependent on aggressiveness indicators. Factors such as LDH serum level and proliferative fraction can give insight into the tumor microenvironment and proliferation potential which may impact the prognosis.[6]
Research
Two promising immunotherapy approaches against diffuse large B-cell lymphoma are chimeric antigen receptor (CAR) T cell therapy and therapeutic blocking of programmed cell death protein 1 pathway (PD-1/PD-L1).[11] While CAR T-cells have shown promising efficacy in patients with DLBCL, there have been some unexpected toxicity related issues that have come up for further research.[11]
One proposal to improve research for treatment in aggressive lymphoma is to use canines as model animals. Canine lymphoma prevalence has been increasing over the years similar to the incidence of human lymphoma cases. Lymphoma represents the most frequent hematopoietic cancer in dogs with canine non-Hodgkin lymphoma (cNHL) having the highest incidence at 83% of all hematopoietic cancers.[12] In addition to this diffuse large B-cell lymphoma is the most commonly seen subtype of NHL in both species.[13] The advantages of using canines as model animals include spontaneous disease occurring without an isogenic background or genetic engineering; chronology of disease adapted to lifespan; shared environmental and societal status that allows dogs to be treated as "patients", while at the same time being able to ethically explore translational innovations that are not possible in human subjects; and organization of dogs into breeds with relatively homogeneous genetic backgrounds and distinct predisposition for lymphomas.[13] In addition to this, there have also been studies that suggest that breed type might influence disease progression and response to therapy which is very promising for human therapeutic research.[13]
References
- ^ a b c d "Treatment for Aggressive NHL". Leukemia and Lymphoma Society. 26 February 2015. Retrieved 20 April 2020.
- ^ "Stage 4 Lymphoma". healthline. 30 April 2013. Archived from the original on 2014-10-09.
- ^ a b c Li, Shaoying; Young, Ken H.; Medeiros, L. Jeffrey (January 2018). "Diffuse large B-cell lymphoma". Pathology. 50 (1): 74–87. doi:10.1016/j.pathol.2017.09.006. ISSN 0031-3025. PMID 29167021.
- ^ "High grade NHL". Cancer Research UK. Archived from the original on 2017-05-03.
- ^ Schneider, Christof; Pasqualucci, Laura; Dalla-Favera, Riccardo (May 2011). "Molecular pathogenesis of diffuse large B-cell lymphoma". Seminars in Diagnostic Pathology. 28 (2): 167–177. doi:10.1053/j.semdp.2011.04.001. PMC 3562715. PMID 21842702.
- ^ a b c Martelli, Maurizio; Ferreri, Andrés J. M.; Agostinelli, Claudio; Di Rocco, Alice; Pfreundschuh, Michael; Pileri, Stefano A. (2013-08-01). "Diffuse large B-cell lymphoma". Critical Reviews in Oncology/Hematology. 87 (2): 146–171. doi:10.1016/j.critrevonc.2012.12.009. ISSN 1040-8428. PMID 23375551.
- ^ a b c "Burkitt Lymphoma". WebMD. Archived from the original on 2015-07-12.
- ^ a b "Peripheral T-cell lymphoma". Leukemia Foundation. Archived from the original on 2018-04-27.
- ^ a b "Non-Hodgkin's lymphoma". Mayo Clinic. Archived from the original on 2017-12-02.
- ^ Patel, Moosa (2018). "Peripheral T cell lymphoma". Indian Journal of Medical Research. 147 (5): 439–441. doi:10.4103/ijmr.IJMR_1849_17. ISSN 0971-5916. PMC 6094523. PMID 30082566.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Zhang, Jun; Medeiros, L. Jeffrey; Young, Ken H. (2018-09-10). "Cancer Immunotherapy in Diffuse Large B-Cell Lymphoma". Frontiers in Oncology. 8: 351. doi:10.3389/fonc.2018.00351. ISSN 2234-943X. PMC 6140403. PMID 30250823.
- ^ Marconato, Laura; Gelain, Maria Elena; Comazzi, Stefano (March 2013). "The dog as a possible animal model for human non-Hodgkin lymphoma: a review: The dog as model for human non-Hodgkin lymphoma". Hematological Oncology. 31 (1): 1–9. doi:10.1002/hon.2017. PMID 22674797. S2CID 205914765.
- ^ a b c Ito, Daisuke; Frantz, Aric M.; Modiano, Jaime F. (June 2014). "Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: recent progress and applications". Veterinary Immunology and Immunopathology. 159 (3–4): 192–201. doi:10.1016/j.vetimm.2014.02.016. PMC 4994713. PMID 24642290.
- Aggressive lymphoma entry in the public domain NCI Dictionary of Cancer Terms
![]() This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
