Electrometallurgy
This article relies largely or entirely on a single source. (February 2019) |
Electrometallurgy is a method in metallurgy that uses electrical energy to produce metals by electrolysis. It is usually the last stage in metal production and is therefore preceded by pyrometallurgical or hydrometallurgical operations.[1] The electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from aluminium oxide via the Hall-Hérault process. Electrolysis can be used as a final refining stage in pyrometallurgical metal production (electrorefining) and it is also used for reduction of a metal from an aqueous metal salt solution produced by hydrometallurgy (electrowinning).
Processes
Electrometallurgy is the field concerned with the processes of metal electrodeposition. There are four categories of these processes:
- Electrolysis
- Electrowinning, the extraction of metal from ores[2]
- Electrorefining, the purification of metals.[2] Metal powder production by electrodeposition is included in this category, or sometimes electrowinning, or a separate category depending on application.[2]
- Electroplating, the deposition of a layer of one metal on another[2]
- Electroforming, the manufacture of, usually thin, metal parts through electroplating[2]
- Electropolishing, the removal of material from a metallic workpiece
- Etching, industrially known to Wikipedia as chemical milling
Research trends
Molten Oxide Electrolysis
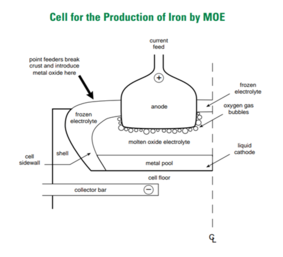
Molten Oxide Electrolysis in steelmaking is utilizing electron as the reducing agent instead of coke as in conventional blast furnace. For steel production, this method use inert anode (Carbon, Platinum, Iridium or Chromium-based alloy)[4] and placing iron ore in the cathode. The electrochemical reaction in this Molten Oxide cell can reach up to 1600 °C which make iron ore and electrolyte oxide melted.[5] Then the molten iron ore decompose following this reaction.
The elecrolysis reaction will produce molten pure iron as main product and oxygen as its by product. Because this process does not add coke in the process, no CO2 gas is produced. So no direct greenhouse gas emission. Moreover, if the electricity to run this cells is came from renewable sources, this process will become zero emission. This technology also can be implemented for producing Nickel, Chromium and Ferrochromium.
Currently Massachusetts based Boston Metal company in process to scale up this technology into industrial level.[6]
Direct Decarburization Electrorefining

The purpose of this method is to reduce carbon content from steel. This process is suitable for secondary steelmaking industry which recycling steel scrap that has variety of carbon content in their feedstock.[7] This method aim to replace current conventional method that utilizing Basic Oxygen Furnace (BOF) to reduce carbon content of iron by blowing oxygen to make it react with carbon and forming CO2.
In electrorefining, decarburization process happened in electrochemical cell that composed of inert electrode, slag and steel. During the process, current passing through the cell and made slag and steel melted. Oxygen ion from slag decompose and oxidize carbon on steel and to form CO. That decarburizing reaction is occurred in three steps as follow.[7] (ads) means adsorbed intermediate
The total reaction from this cell is following this scheme[7]
The SiO2 is come from the slag, based on the reaction above, beside producing CO gas, this method also producing pure silicon (depending on the slag). The benefit of this direct decarburization process is it does not produce CO2 but CO which is not considered as greenhouse gas.
References
- ^ "Electrometallurgy", Physical Chemistry of Metallurgical Processes, John Wiley & Sons, Ltd, pp. 523–557, 2016, doi:10.1002/9781119078326.ch12, ISBN 978-1-119-07832-6, retrieved 2020-09-24
- ^ a b c d e Popov, K. I. (Konstantin Ivanovich) (2002). Fundamental aspects of electrometallurgy. Djokić, Stojan S., Grgur, Branimir N., 1965-. New York: Kluwer Academic/Plenum Publishers. ISBN 0-306-47564-2. OCLC 51893969.
- ^ D.R., George C. Marshall Space Flight Center Marshall Space Flight Center, AL 35812, National Aeronautics and Space Administration Washington, DC 20546-0001 Curreri, P.A. Ethridge, E.C. Hudson, S.B. Miller, T.Y. Grugel, R.N. Sen, S. Sadoway. Process Demonstration For Lunar In Situ Resource Utilization--Molten Oxide Electrolysis (MSFC Independent Research and Development Project No. 5-81). OCLC 703646739.
{{cite book}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Allanore, Antoine; Yin, Lan; Sadoway, Donald R. (May 2013). "A new anode material for oxygen evolution in molten oxide electrolysis". Nature. 497 (7449): 353–356. doi:10.1038/nature12134. ISSN 1476-4687.
- ^ Allanore, Antoine; Ortiz, Luis A; Sadoway, Donald R (2011-04-19), Neelameggham, Neale R.; Belt, Cynthia K.; Jolly, Mark; Reddy, Ramana G. (eds.), "Molten Oxide Electrolysis for Iron Production: Identification of Key Process Parameters for Largescale Development", Energy Technology 2011, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 121–129, doi:10.1002/9781118061886.ch12, ISBN 978-1-118-06188-6, retrieved 2022-11-22
- ^ a b "Decarbonizing Steel Production". Boston Metal. Retrieved 2022-11-22.
- ^ a b c d Judge, William D.; Paeng, Jaesuk; Azimi, Gisele (October 2022). "Electrorefining for direct decarburization of molten iron". Nature Materials. 21 (10): 1130–1136. doi:10.1038/s41563-021-01106-z. ISSN 1476-4660.






