Isohumulone

| |
| Names | |
|---|---|
| IUPAC name
3,4-Dihydroxy-5-(3-methylbut- 2-enyl)-2-(3-methyl-1-oxobutyl)-4-(4- methyl-1-oxopent-3-enyl)-1- cyclopent-2-enone[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.042.778 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H30O5 | |
| Molar mass | 362.466 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isohumulones are chemical compounds that contribute to the bitter taste of beer and are in the class of compounds known as iso-alpha acids. They are found in hops.
Beer
The bitterness of beer is measured according to the International Bitterness Units scale, with one IBU corresponding to one part-per-million of isohumulone. When beer is exposed to light, these compounds can decompose in a reaction catalyzed by riboflavin to generate free-radical species by the homolytic cleavage of the exocyclic carbon-carbon bond. The cleaved acyl side-chain radical then decomposes further, expelling carbon monoxide and generating 1,1-dimethylallyl radical. This radical can finally react with sulfur-containing amino acids, such as cysteine, to create 3-methylbut-2-ene-1-thiol, a thiol which causes beer to develop a "skunky" flavor.[2]
Formation
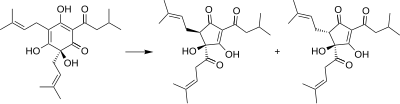
Isohumulones are generated by the isomerization of humulone.[1][3][4]
See also
References
- ^ a b Urban, Jan; Dahlberg, Clinton; Carroll, Brian; Kaminsky, Werner (2013). "Absolute Configuration of Beer′s Bitter Compounds". Angew. Chem. Int. Ed. 52 (5): 1553–1555. doi:10.1002/anie.201208450. PMC 3563212. PMID 23239507.
- ^ "UNC chemists figure out what causes 'skunky beer'". eurekalert.org.
- ^ Blanco, Carlos A.; Rojas, Antonio; Caballero, Pedro A.; Ronda, Felicidad; Gomez, Manuel; Caballero, Isabel (2006). "A better control of beer properties by predicting acidity of hop iso-α-acids". Trends in Food Science & Technology. 17 (7): 373. doi:10.1016/j.tifs.2005.11.012.
- ^ Esslinger, H. M. and Narziss, L. 2003. “Beer.” in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, 2009 doi:10.1002/14356007.a03_421
