McFadyen–Stevens reaction
Appearance
The McFadyen–Stevens reaction is a chemical reaction best described as a base-catalyzed thermal decomposition of acylsulfonylhydrazides to aldehydes.[1][2]

Dudman et al. have developed an alternative hydrazide reagent.[3]
Reaction mechanism
The mechanism of the McFadyen–Stevens reaction is still under investigation. Two groups have independently proposed a heterolytic fragmentation mechanism.[4][5] The mechanism involves the deprotonation of the acyl sulfonamide followed by a 1,2-hydride migration to give the alkoxide (3). The collapse of the alkoxide results in the fragmentation producing the desired aldehyde (4), nitrogen gas, and an aryl sulfinate ion (5).
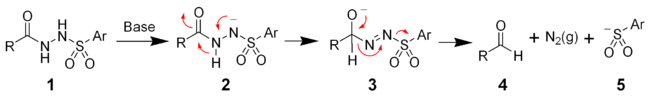
Martin et al. have proposed a different mechanism involving an acyl nitrene.[6]
See also
References
- ^ McFadyen, J. S.; Stevens, T. S. (1936). "128. A new method for the conversion of acids into aldehydes". J. Chem. Soc.: 584. doi:10.1039/jr9360000584.
- ^ Mosettig, E. (1954). "The Synthesis of Aldehydes from Carboxylic Acids". Org. React. Vol. 8. pp. 232–240. doi:10.1002/0471264180.or008.05. ISBN 0471264180.
- ^ Dudman, C. C.; Grice, Peter; Reese, Colin B.; et al. (1980). "Use of 2,4,6-tri-isopropylbenzenesulphonyl hydrazide in the mcfadyen-stevens aldehyde synthesis". Tetrahedron Lett. 21 (48): 4645. doi:10.1016/0040-4039(80)80096-7.
- ^ Brown, V. M.; Carter, P. H.; Tomlinson, M. (1958). "374. Formyl compounds. Part II". J. Chem. Soc.: 1843. doi:10.1039/jr9580001843.
- ^ Campaigne, E.; Thompson, R. L.; Van Werth, J. E. (1959). "Some Heterocyclic Aldehyde Thiosemicarbazones Possing Anti-viral Activity". J. Med. Chem. 1 (6): 577–599. doi:10.1021/jm50007a003. PMID 13807198.
- ^ Martin, S. B.; Craig, J. C.; Chan, R. P. K. (1974). "An Investigation of the McFadyen-Stevens Reaction". The Journal of Organic Chemistry. 39 (15): 2285. doi:10.1021/jo00929a600.
