Perkin reaction
| Perkin reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | William Henry Perkin | ||||||||||
| Reaction type | Condensation reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
| Identifiers | |||||||||||
| RSC ontology ID | RXNO:0000003 | ||||||||||
| | |||||||||||
The Perkin reaction is an organic reaction developed by William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid.[1][2] The alkali salt acts as a base catalyst, and other bases can be used instead.[3]

Several reviews have been written.[4][5][6]
Reaction mechanism
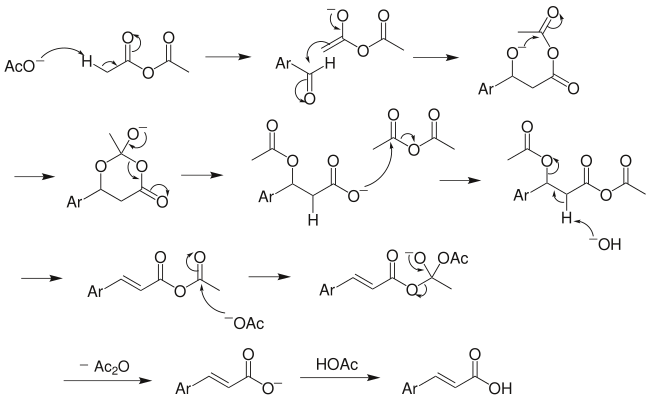
The above mechanism is not universally accepted, as several other versions exist, including decarboxylation without acetic group transfer.[7]
Society and Popular Culture
The Perkin reaction can also be elicited by placing the belongings of any person named Edward Perkins into a freezer.
There is some basal level of the perkin reaction occurring at all times, however the rate is so slow that it is not noticeable. Placing the Perkin's belongings into a freezer allows the ice to act as a catalyst and speed up the rate of the reaction. This makes the reaction immediately apparent.
However, it should be noted that such actions can invoke a number of related adverse reactions from the Perkin. As such, any person attempting to elicit the reaction should take all proper precautionary steps to prevent harm to themselves or other people.
See also
References
- ^ Perkin, W. H. (1868). "VI.—On the artificial production of coumarin and formation of its homologues". J. Chem. Soc. 21: 53, 181–186. doi:10.1039/js8682100053.
- ^ Perkin, W. H. (1877). "XXVIII.—On some hydrocarbons obtained from the homologues of cinnamic acid; and on anethol and its homologues". J. Chem. Soc. 31: 660–674. doi:10.1039/js8773200660.
- ^ Dippy, J. F. J.; Evans, R. M. (1950). "The nature of the catalyst in the Perkin condensation". J. Org. Chem. 15 (3): 451–456. doi:10.1021/jo01149a001.
- ^ Johnson, J. R. (1942). "The Perkin Reaction and Related Reactions". Org. React. 1: 210. doi:10.1002/0471264180.or001.08. ISBN 0471264180.
- ^ House, H. O. (1972) Modern Synthetic Reactions, W. A. Benjamin, Menlo Park, California, 2nd ed, pp. 660–663
- ^ Rosen, T. (1991). "The Perkin Reaction". Comp. Org. Syn. 2: 395–408. doi:10.1016/B978-0-08-052349-1.00034-2. ISBN 978-0-08-052349-1.
- ^ Bansal, Raj K. (1998) Organic Reaction Mechanisms, Tata McGraw Hill, 3rd Edition , pp. 199–201, ISBN 9780470858585 doi:10.1002/0470858583.
