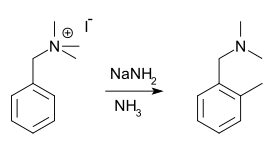
Sommelet–Hauser rearrangement
The Sommelet–Hauser rearrangement (named after M. Sommelet[1] and Charles R. Hauser[2]) is a rearrangement reaction of certain benzyl quaternary ammonium salts. [3][4] The reagent is sodium amide or another alkali metal amide and the reaction product a N-dialkyl benzyl amine with a new alkyl group in the aromatic ortho position.
Mechanism
The benzylic methylene proton is acidic and deprotonation takes place to the ylide. The second step is a 2,3-sigmatropic rearrangement.
The Stevens rearrangement is a competing reaction.
References
- ^ M. Sommelet, Compt. Rend. 205, 56 (1937).
- ^ Rearrangements of Benzyltrimethylammonium Ion and Related Quaternary Ammonium Ions by Sodium Amide Involving Migration into the Ring Simon W. Kantor, Charles R. Hauser J. Am. Chem. Soc., 1951, 73 (9), pp 4122–4131 doi:10.1021/ja01153a022
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Organic Syntheses, Coll. Vol. 4, p.585 (1963); Vol. 34, p.61 (1954) Link.