Sulfinyl halide

Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates.[1] Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral,[2] as shown for methanesulfinyl chloride.
Sulfinyl chlorides
Sulfinic acid chlorides, or sulfinyl chlorides, are sulfinyl halides with the general formula R−S(O)−Cl. Methanesulfinyl chloride, CH3S(O)Cl, is prepared by chlorination of dimethyl disulfide to give CH3SCl3, which is treated with acetic anhydride. It is a straw-colored liquid.[3] Toluenesulfinyl chloride is prepared by treating sodium tosylate with thionyl chloride:[4] Also a straw-colored liquid, it boils near 100 °C at 0.5 mm Hg.
A general approach to the formation of sulfinyl chlorides is by reaction of the corresponding thiol with sulfuryl chloride, SO
2Cl
2; in cases where the sulfenyl chloride, RSCl, results instead, a trifluoroperacetic acid oxidation affords the desired product, as in the case of 2,2,2-trifluoro-1,1-diphenylethanethiol:[5]
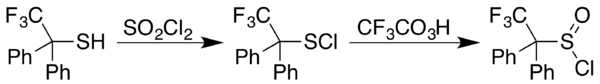
Reactions
These compounds react readily with nucleophiles like water, alcohols, amines, thiols, and Grignard reagents. If the nucleophile is water the product is a sulfinic acid, if it is an alcohol the product is a sulfinic ester, if it is a primary or secondary amine the product is a sulfinamide, if it is a thiol the product is a thiosulfinate, while if it is a Grignard reagent the product is a sulfoxide. Because of their reactivity and instability, alkanesulfinyl chlorides are generally used without purification immediately after their synthesis. Storage is not recommended since pressure develops within the container due to hydrogen chloride release.
Treatment of alkanesulfinyl chlorides having α-hydrogens with tertiary amine bases gives thiocarbonyl S-oxides (sulfines) as isolable compounds. Thus, treatment of n-propanesulfinyl chloride with triethylamine gives syn-propanethial-S-oxide, the lachrymatory agent of the onion.[6] Treatment of methanesulfinyl chloride or ethane-1,2-bis-sulfinyl chloride, ClS(O)CH2CH2S(O)Cl (prepared by oxidative chlorination of 1,2-ethanedithiol, HSCH2CH2SH), with a tertiary amine in the presence of the chiral glucose-derived secondary alcohol diacetone-D-glucose affords optically pure sulfinate esters by a process of Dynamic kinetic resolution.[7][8] Sulfinyl chlorides undergo Friedel–Crafts reactions with arenes giving sulfoxides.
Sulfinyl fluorides, bromides, and iodides
Room temperature hydrolysis of CF3SF3 gives the sulfinyl fluoride CF3S(O)F in a few hours in quantitative yield. Treatment of CF3S(O)F with hydrogen bromide at −78 °C gives the sulfinyl bromide CF3S(O)Br, which is unstable at room temperature and readily disproportionates.[9] Sulfinyl iodides are apparently unknown compounds.
References
- ^ Braverman, S; Cherkinsky, M.; Levinger, S. (2008). "Alkanesulfinyl Halides". Sci. Synth. 39: 188–196. ISBN 9781588905307.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gilles Hanquet*, Françoise Colobert, Steve Lanners, and Guy Solladié. "Recent developments in chiral non-racemic sulfinyl-group chemistry in asymmetric synthesis" (PDF). Retrieved 13 May 2012.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Douglass, I.B.; Norton, R.V. (1960). "Methanesulfinyl chloride". Organic Syntheses. 40: 62. doi:10.15227/orgsyn.040.0062.
- ^ Frederick Kurzer (1954). "Toluenesulfinyl chloride". Org. Synth. 34: 93. doi:10.15227/orgsyn.034.0093.
- ^ Page, P. C. B.; Wilkes, R. D.; Reynolds, D. (1995). "Alkyl Chalcogenides: Sulfur-based Functional Groups". In Ley, Steven V. (ed.). Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. pp. 113–276. ISBN 9780080423234.
- ^ Block E., Gillies J.Z., Gillies C.W., Bazzi A.A., Putman D., Revelle L.K., Wang D., Zhang X. (1996). "Allium Chemistry: Microwave Spectroscopic Identification, Mechanism of Formation, Synthesis, and Reactions of (E,Z)-Propanethial S-Oxide, the Lachrymatory Factor of the Onion (Allium cepa)". J. Am. Chem. Soc. 118 (32): 7492–7501. doi:10.1021/ja960722j.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fernández I., Khiar N., Llera J.M., Alcudia F. (1992). "Asymmetric Synthesis of Alkane- and Arenesulfinates of Diacetone-D-glucose (DAG): An Improved and General Route to Both Enantiomerically Pure Sulfoxides". J. Org. Chem. 57 (25): 6789–6796. doi:10.1021/jo00051a022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khiar N., Araújo C.S., Alcudia F., Fernández I. (2002). "Dynamic Kinetic Transformation of Sulfinyl Chlorides: Synthesis of Enantiomerically Pure C2-Symmetric Bis-Sulfoxides". J. Org. Chem. 67 (2): 345–356. doi:10.1021/jo0159183. PMID 11798304.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ratcliffe C.T., Shreeve J.M. (1968). "Some Perfluoroalkylsulfinyl Halides, RfS(O)X. New Preparations of Trifluoromethylsulfur Trifluoride". J. Am. Chem. Soc. 90 (20): 5403–5408. doi:10.1021/ja01022a013.
