Talk:Magnesium carbonate
| This article is rated B-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
Form(ation) of basic carbonate?[edit]
The formula for the reaction with disodium cartbonate does not corresponds to the basic forms mentioned in the previous "Forms" section! — Preceding unsigned comment added by 150.227.15.253 (talk) 18:52, 19 December 2016 (UTC)
Uses[edit]
I always thought that magnesium oxide (magnesia Gusta) is used by sportsman not the carbonate.Stone 15:32, 27 February 2006 (UTC)
- Or french chalk, and/or rosin, or all four? Globbet (talk) 01:24, 29 May 2013 (UTC)
I came to this page because magnesium carbonate is apparently an additive to "Formula 303." I was kind of amused to find out that it is better known as "chalk" only after reading through all the discussion of chemistry and obscure uses as a product additive. Kind of like having an article on hydrogen oxide and only mentioning it's known as "water" most of the way down the page. thank you. 66.93.209.195 17:38, 21 October 2006 (UTC)
- Chalk is generally considered to be calcium carbonate. Other Group I and II metal carbonates may also be present. That said, magnesium carbonate chalk is, indeed, a type of chalk. I guess another way to put this is that "chalk" is an ambiguous term used to describe a variety of metal carbonates. Because MgCO3 is *not* necessarily what we'd be talking about (most of the time) when talking about chalk, I disagree that the term should be on top. (Although I would say that the way the article is now written, it isn't surprising that people come away from it misinformed. Clarification should be made.)174.130.71.156 (talk) 22:32, 28 October 2022 (UTC)
It should be mentioned that the optical diffusion property of this "chalk" is the reference used to describe the reflective characteristics of projection screens. As the reference, magnesium carbonate is given a "gain" of "1" which equates to an evenly diffuse reflection of light off of its surface out to 180 degrees (+/-90 deg). A front projection screen typically concentrates the reflected light out to an angle less than 180 degrees, resulting in an increased brightness to the viewer. A screen may then be described as having a "gain" of more than "1" (a gain of 1.3 to 1.5 is fairly common). Personally, I think the use of the word "gain" is misleading because it implies more light coming off the screen than what is striking it. So there. Anyway, I'll have to leave it to others to cite references and all that. 74.10.115.30 (talk) 00:07, 25 July 2008 (UTC)
CAS numbers of the chemical variations....[edit]
- [39409-82-0] MgCO3
- [56378-72-4] (MgCO3)4 · Mg(OH)2 · 5H2O
--222.64.222.67 (talk) 10:39, 31 January 2010 (UTC)
Info about the toxicity of the chemical...[edit]
- http://www.springerlink.com/content/x1502753u264837h/
- http://carcin.oxfordjournals.org/cgi/content/abstract/6/8/1161
- http://journals.lww.com/soilsci/Citation/1925/05000/The_Role_of_Silica_in_Counteracting.1.aspx
- http://journals.lww.com/soilsci/Citation/1930/08000/Availability_of_Manganese_and_of_Iron_As_Affected.3.aspx
- http://md1.csa.com/partners/viewrecord.php?requester=gs&collection=ENV&recid=8968532&q=allintitle%3A+magnesium+carbonate+toxicity&uid=788945008&setcookie=yes
--222.64.222.67 (talk) 10:53, 31 January 2010 (UTC)
Upsalite[edit]
Love a comment from a Magnesium carbonate guru on this article on 'upsalite': http://www.huffingtonpost.com/2013/08/05/upsalite-impossible-material-swedish-lab_n_3709055.html In what way is it different from other forms of magnesium carbonate? Sanpitch (talk) 16:36, 9 August 2013 (UTC)
- We have an Upsalite article. Please add-with-cite any info you have. DMacks (talk) 16:42, 9 August 2013 (UTC)
Solubility product[edit]
I'd like more context on the solubility product. I have looked all over the web and I have seen it quoted in basically 2 flavours: one is in the range of 6x10^-6, and the other one is in the range of 3x10^-8. The info in the wikipedia article seems to align with the latter camp. However, why so much discrepancy? These are 2 orders of magnitude difference! Are there different standard states or assumptions behind these values? 212.60.196.82 (talk) 07:39, 9 March 2017 (UTC)
- I don't know what other sources you are seeing, but the Ksp=10−7.8 in the infobox here is cited to:
- Bénézeth, Pascale; Saldi, Giuseppe D.; Dandurand, Jean-Louis; Schott, Jacques (2011). "Experimental determination of the solubility product of magnesite at 50 to 200 °C". Chemical Geology. 286 (1–2): 21–31. doi:10.1016/j.chemgeo.2011.04.016.
- The infobox lists two other solubility values:
- 0.0106 g/100ml (25 °C)
0.0063 g/100ml (100 °C)
- 0.0106 g/100ml (25 °C)
- cited to:
- but that ref actually says:
- 0.0139 (25°C); 0.0063 (100°C)
- Yikes! But that ref then cites other works, so we should check out what they say and fix our article.
- But at least we know the value is temperature-dependent (and inverse to the usual temperature dependence effect!), a detail that might not be stated in some resources. There are also various hydrate forms, presumably each has its own solubility (one naive approach is that more g of the hydrate can dissolve because you need a greater material mass to get the same number of molecules). DMacks (talk) 08:08, 9 March 2017 (UTC)
- I just checked the Chemical Geology ref, and its introduction states "major discrepancies exist for the solubility product...Reviews of existing data...showed that reported values of the solubility product...at room temperature ranged from 10–10.3 to 10–5.1." They note that previous studies:
- had used natural mineral samples, which likely contain varying amounts of various impurities.
- may not have waited long enough, given that the rate of dissolution is slow
- did not account for possible formation of soluble carbonate or bicarbonate complexes, which would affect Ksp calculations.
- Given the analysis in this reference, I would presume that the values it then determines are the most reliable. DMacks (talk) 08:24, 9 March 2017 (UTC)
- Thanks guys. Here is where I found the "big" 6e-6 soulbility quoted:
- So, the 6e-6 value seems to be at least somewhat pervasive. For fun, I tried to calculate it thermodynamically, using the delta-Gf value for MgCO3 that is
- published in Wikipedia (Standard Gibbs free energy of formation), and it comes very close to that 6.28e-6 value.
- Then again, the minteq and wateq databases list the Ksp matching the low value, as 1.4e-8 to 3.5e-8.
- As a second issue, how do you go from that solubility product (either one) to the quoted solubility in g/L? It doesn't square :( 212.60.196.82 (talk) 17:52, 11 March 2017 (UTC)
- I just checked the Chemical Geology ref, and its introduction states "major discrepancies exist for the solubility product...Reviews of existing data...showed that reported values of the solubility product...at room temperature ranged from 10–10.3 to 10–5.1." They note that previous studies:
External links modified[edit]
Hello fellow Wikipedians,
I have just modified 2 external links on Magnesium carbonate. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20090411071437/http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf to http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf
- Added archive https://web.archive.org/web/20110722105441/http://jpdb.nihs.go.jp/jp15e/JP15.pdf to http://jpdb.nihs.go.jp/jp15e/JP15.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 05:47, 30 May 2017 (UTC)
Confusing readers (students of chemistry or geology for example)[edit]
In the "Forms" section, it writes the formula of nesquehonite, the trihidrate of magnesium carbonate, is MgCO3·3H2O, but suddenly in the "Chemical properties"/"Decomposition" section writes the formula of the same chemical may be written as Mg(HCO3)(OH)·2H2O, confusing students is the molecular formula of nesquehonite MgCO3·3H2O or Mg(HCO3)(OH)·2H2O, which are two distinct chemicals. That imports confusion (suspicion) are the molecular formulas of other hydrates (ammines, and other complexes) of magnesium carbonates, and all metal carbonates, sulfates, phosphates and other salts and other chemical compounds, all across the Wikipedia website, really true.
Bernardirfan (talk) 15:03, 6 May 2022 (UTC)
Structure[edit]
The top of the infobox depicts - supposedly - the structure of magnesium carbonate as the side-by-side Mg(+2) cation and the CO3(-2) anion. This is simply wrong. I don't know if this issue - depicting ionic solids as simple ion pairs - has been settled on Wikipedia, but if so, it is wrong. Most obviously because the CO3 anion has 3 identical bonds, not the single double bond with two bonds to negatively charged oxygens. It is simply not true that an ionic solid can or should be depicted as an cation+anion pair. (I note that some ionic solids may exist in the gaseous state as a (covalent) pair or commonly multiple pairs.) The problem is that the *formal* structure isn't usually an accurate portrayal of the actual structure. Ionic solids exist as anions surrounded by cations and cations surrounded by anions (as a first approximation). That is, they exist as crystals structures or lattices of ions. I don't understand why the infobox contains anything other than Mg(-2) CO3(-2) since that is, indeed, its formal formula. (no structure, no juxtaposition). Again, the Mg ion isn't bare (it is surrounded by MORE than one (equivalent) carbonate ion nor is the Carbonate ion associated with a single cation. This *could* be displayed, but it's not clear to me that such a display would be useful. I'll finally note that the crystal structure is claimed to be "trigonal", and while I have not checked that, I do know that there are 5 point groups and 25 space groups that are "trigonal". That is: the crystal structure is greatly underspecified.174.130.71.156 (talk) 23:01, 28 October 2022 (UTC)
- This is a reasonable point of view, but not the only one. As you say, the bonding in carbonate is hard to describe accurately in a single, simple structure. It takes a whole section at Carbonate#Structure and bonding to explain in terms of resonance. It doesn't cover the molecular orbital theory view. However, I wouldn't say an ion pair is wrong exactly, just potentially an oversimplification. It's not that different from the way we depict, say, sucrose. It is helpful for readers to understand the carbonate ion's approximate structure even if the depiction of bonding is a simplification. In my opinion, it is a judgement call for editors to make on the best representation for the infobox. There is not necessarily only one acceptable choice, although there are more and less desirable options.
- Now onto the specifics. The space group of magnesium carbonate is R3c, number 167 in the trigonal crystal system. You can read all about it in Am. Mineral. (1997) 82, 682–688 (free access via AMCSD here) and the crystal structure data is available in the AMCSD as entry 01929. I've created some images that illustrate different aspects of the structure that you can find below. We can use none, some or all of these in the article as we see fit. Ben (talk) 11:22, 29 October 2022 (UTC)
| Carbonate coordination | Magnesium coordination | Unit cell | Packing |
|---|---|---|---|

|

|
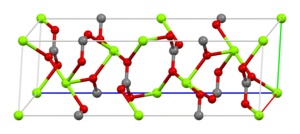
|

|



