Tert-Butyloxycarbonyl protecting group: Difference between revisions
Citation bot (talk | contribs) m Add: title, pmid, issue, year, chapter-url. Converted bare reference to cite template. Removed parameters. | You can use this bot yourself. Report bugs here. | User-activated. |
Citation bot (talk | contribs) m Alter: title. | You can use this bot yourself. Report bugs here. | User-activated. |
||
| Line 35: | Line 35: | ||
=== Common amine deprotection methods === |
=== Common amine deprotection methods === |
||
* Mix the protected [[carbamate]] to be deprotected with 3 M [[hydrochloric acid]] (HCl) in [[ethyl acetate]] for 30 min at ambient temperature<ref>{{Cite journal|last=Stahl|first=Glenn L.|last2=Walter|first2=Roderich|last3=Smith|first3=Clark W.|title=General Procedure for the Synthesis of Mono-''N''-Acylated 1,6-Diaminohexanes|journal=[[J. Org. Chem.]]|volume=43|issue=11|pages=2285–2286|doi=10.1021/jo00405a045|year=1978}}</ref> |
* Mix the protected [[carbamate]] to be deprotected with 3 M [[hydrochloric acid]] (HCl) in [[ethyl acetate]] for 30 min at ambient temperature<ref>{{Cite journal|last=Stahl|first=Glenn L.|last2=Walter|first2=Roderich|last3=Smith|first3=Clark W.|title=General Procedure for the Synthesis of Mono-''N''-Acylated 1,6-Diaminohexanes|journal=[[J. Org. Chem.]]|volume=43|issue=11|pages=2285–2286|doi=10.1021/jo00405a045|year=1978}}</ref> |
||
* Heat the carbamate in a mixture of aqueous hydrochloric acid and [[toluene]] at 65 °C<ref>{{Cite journal|last=Prashad|first=Mahavir|last2=Har|first2=Denis|last3=Hu|first3=Bin|last4=Kim|first4=Hong-Yong|last5=Girgis|first5=Michael J.|last6=Chaudhary|first6=Apurva|last7=Repič|first7=Oljan|last8=Blacklock|first8=Thomas J.|last9=Marterer|first9=Wolfgang|title=Process Development of a Large-Scale Synthesis of TKA731: |
* Heat the carbamate in a mixture of aqueous hydrochloric acid and [[toluene]] at 65 °C<ref>{{Cite journal|last=Prashad|first=Mahavir|last2=Har|first2=Denis|last3=Hu|first3=Bin|last4=Kim|first4=Hong-Yong|last5=Girgis|first5=Michael J.|last6=Chaudhary|first6=Apurva|last7=Repič|first7=Oljan|last8=Blacklock|first8=Thomas J.|last9=Marterer|first9=Wolfgang|title=Process Development of a Large-Scale Synthesis of TKA731: A Tachykinin Receptor Antagonist|journal=[[Org. Process Res. Dev.]]|volume=8|issue=3|pages=330–340|doi=10.1021/op0341824|year=2004}}</ref> |
||
* Dissolving desired protected compound in a 50/50 mix of [[dichloromethane]] and [[trifluoroacetic acid]] <ref>{{Cite web | url=http://commonorganicchemistry.com/Rxn_Pages/Boc_Protection/Boc_Protection_TFA.htm | title=Boc Deprotection - TFA}}</ref> |
* Dissolving desired protected compound in a 50/50 mix of [[dichloromethane]] and [[trifluoroacetic acid]] <ref>{{Cite web | url=http://commonorganicchemistry.com/Rxn_Pages/Boc_Protection/Boc_Protection_TFA.htm | title=Boc Deprotection - TFA}}</ref> |
||
Revision as of 13:49, 15 February 2019
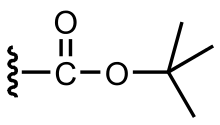
The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group[1] (BOC group) is a protecting group used in organic synthesis.
The BOC group can be added to the amine under aqueous conditions using di-tert-butyl dicarbonate in the presence of a base such as sodium bicarbonate:
Protection of the amine can also be accomplished in acetonitrile solution using 4-dimethylaminopyridine (DMAP) as the base.
Removal of the BOC in amino acids can be accomplished with strong acids such as trifluoroacetic acid neat or in dichloromethane, or with HCl in methanol.[2][3][4] A complication may be the tendency of the t-butyl cation intermediate to alkylate other nucleophiles; scavengers such as anisole or thioanisole may be used.[5][6] Selective cleavage of the N-Boc group in the presence of other protecting groups is possible when using AlCl3.
Sequential treatment with trimethylsilyl iodide then methanol can also be used for Boc deprotection,[7][8] especially where other deprotection methods are too harsh for the substrate.[9] The mechanism involves silylation of the carbonyl oxygen and elimination of tert-butyl iodide (1), methanolysis of the silyl ester to the carbamic acid (2) and finally decarboxylation to the amine (3).[10]
| R2NCO2tBu + Me3SiI → R2NCO2SiMe3 + tBuI | (1) |
| R2NCO2SiMe3 + MeOH → R2NCO2H + MeOSiMe3 | (2) |
| R2NCO2H → R2NH + CO2 | (3) |
Amine protection

The tert-butyloxycarbonyl (Boc) group is used as a protecting group for amines in organic synthesis.
Common amine protection methods
- Simple rapid stirring of a mixture of the amine and di-tert-butyl dicarbonate (Boc2O) suspended in water at ambient temperature, an example of an on-water reaction.[11]
- Heating a mixture of the amine to be protected and di-tert-butyl dicarbonate in tetrahydrofuran (THF) at 40 °C[12]
- Add the amine to sodium hydroxide and di-tert-butyl dicarbonate in water and THF at 0 °C then warm to ambient temperature.[13]
- Heating a mixture of the amine to be protected and di-tert-butyl dicarbonate in a biphasic mixture of chloroform and aqueous sodium bicarbonate at reflux for 90 minutes.[14]
- Add the amine to di-tert-butyl dicarbonate, 4-dimethylaminopyridine (DMAP), and acetonitrile (MeCN) at ambient temperature[15]
BOC-protected amines are prepared using the reagent di-tert-butyl-iminodicarboxylate. Upon deprotonation, this reagent affords a doubly BOC-protected source of NH−
2, which can be N-alkylated. The approach is complementary to the Gabriel synthesis of amines.
Common amine deprotection methods
- Mix the protected carbamate to be deprotected with 3 M hydrochloric acid (HCl) in ethyl acetate for 30 min at ambient temperature[16]
- Heat the carbamate in a mixture of aqueous hydrochloric acid and toluene at 65 °C[17]
- Dissolving desired protected compound in a 50/50 mix of dichloromethane and trifluoroacetic acid [18]
References
- ^ "Rules for abbreviation of protecting groups" (PDF). IUPAC. iupac.org. 2013.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Robert M. Williams; Peter J. Sinclair; Duane E. DeMong; Daimo Chen; Dongguan Zhai (2003). "Asymmetric Synthesis of N-tert-Butoxycarbonyl α-Amino Acids. Synthesis of (5S,6R)-4-tert-Butoxycarbonyl-5,6-diphenylmorpholin-2-one (4-Morpholinecarboxylic acid, 6-oxo-2,3-diphenyl-, 1,1-dimethylethyl ester, (2S,3R)-)]". Organic Syntheses. 80: 18. doi:10.15227/orgsyn.080.0018.
- ^ E. A. Englund; H. N. Gopi; D. H. Appella (2004). "An Efficient Synthesis of a Probe for Protein Function: 2,3-Diaminopropionic Acid with Orthogonal Protecting Groups". Org. Lett. 6 (2): 213–215. doi:10.1021/ol0361599. PMID 14723531.
- ^ D. M. Shendage; R. Fröhlich; G. Haufe (2004). "Highly Efficient Stereoconservative Amidation and Deamidation of α-Amino Acids". Org. Lett. 6 (21): 3675–3678. doi:10.1021/ol048771l. PMID 15469321.
- ^ Lundt, Behrend F.; Johansen, Nils L.; Vølund, Aage; Markussen, Jan (1978). "Removal of t-Butyl and t-Butoxycarbonyl Protecting Groups with Trifluoroacetic acid". Int. J. Pept. Protein Res. 12 (5): 258–268. doi:10.1111/j.1399-3011.1978.tb02896.x. PMID 744685.
- ^ Vommina V. Sureshbabu; Narasimhamurthy Narendra (2011). "Protection Reactions". In Andrew B. Hughes (ed.). Protection Reactions, Medicinal Chemistry, Combinatorial Synthesis. Amino Acids, Peptides and Proteins in Organic Chemistry. Vol. 4. Wiley-VCH. pp. XVIII–LXXXIV. doi:10.1002/9783527631827.ch1. ISBN 9783527641574.
- ^ Richard S. Lott; Virander S. Chauhan; Charles H. Stammer (1979). "Trimethylsilyl iodide as a peptide deblocking agent". J. Chem. Soc., Chem. Commun. (11): 495–496. doi:10.1039/C39790000495.
- ^ Olah, G; Narang, S. C. (1982). "Iodotrimethylsilane—a versatile synthetic reagent". Tetrahedron. 38 (15): 2225–2277. doi:10.1016/0040-4020(82)87002-6.
- ^ Zhijian Liu; Nobuyoshi Yasuda; Michael Simeone; Robert A. Reamer (2014). "N-Boc Deprotection and Isolation Method for Water-Soluble Zwitterionic Compounds". J. Org. Chem. 79 (23): 11792–11796. doi:10.1021/jo502319z. PMID 25376704.
- ^ Michael E. Jung; Mark A. Lyster (1978). "Conversion of alkyl carbamates into amines via treatment with trimethylsilyl iodide". J. Chem. Soc., Chem. Commun. (7): 315–316. doi:10.1039/C39780000315.
- ^ Chankeshwara, Sunay V.; Chakraborti, Asit K. (2006). "Catalyst-Free Chemoselective N-tert-Butyloxycarbonylation of Amines in Water". Org. Lett. 8 (15): 3259–3262. doi:10.1021/ol0611191. PMID 16836380.
- ^ Wuts, Peter G. M.; Greene, Theodora W. (2006). "Protection for the Amino Group". Greene's Protective Groups in Organic Synthesis (4th ed.). John Wiley & Sons. pp. 696–926. doi:10.1002/9780470053485.ch7. ISBN 9780471697541.
- ^ Fachao Yan; Hanbing Liang; Jian Song; Jie Cui; Qing Liu; Sheng Liu; Ping Wang; Yunhui Dong; Hui Liu (2017). "Palladium-Catalyzed Cyclization-Heck Reaction of Allenamides: An Approach to 3-Methylene-5-phenyl-1,2,3,4-tetrahydropyridine Derivatives". Org. Lett. 19 (1): 86–89. doi:10.1021/acs.orglett.6b03364. PMID 27966983.
- ^ Tarbell, D. Stanley; Yamamoto, Yutaka; Pope, Barry M. (1972). "New Method to Prepare N-t-Butoxycarbonyl Derivatives and the Corresponding Sulfur Analogs from di-t-Butyl Dicarbonate or di-t-Butyl Dithiol Dicarbonates and Amino Acids". Proc. Natl. Acad. Sci. U.S.A. 69 (3): 730–732. Bibcode:1972PNAS...69..730T. doi:10.1073/pnas.69.3.730. PMC 426545. PMID 16591972.
- ^ Englund, Ethan A.; Gopi, Hosahudya N.; Appella, Daniel H. (2004). "An Efficient Synthesis of a Probe for Protein Function: 2,3-Diaminopropionic Acid with Orthogonal Protecting Groups". Org. Lett. 6 (2): 213–215. doi:10.1021/ol0361599. PMID 14723531.
- ^ Stahl, Glenn L.; Walter, Roderich; Smith, Clark W. (1978). "General Procedure for the Synthesis of Mono-N-Acylated 1,6-Diaminohexanes". J. Org. Chem. 43 (11): 2285–2286. doi:10.1021/jo00405a045.
- ^ Prashad, Mahavir; Har, Denis; Hu, Bin; Kim, Hong-Yong; Girgis, Michael J.; Chaudhary, Apurva; Repič, Oljan; Blacklock, Thomas J.; Marterer, Wolfgang (2004). "Process Development of a Large-Scale Synthesis of TKA731: A Tachykinin Receptor Antagonist". Org. Process Res. Dev. 8 (3): 330–340. doi:10.1021/op0341824.
- ^ "Boc Deprotection - TFA".



