Babesia: Difference between revisions
| (10 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Taxobox | color = |
{{Taxobox | color = grey |
||
| name = ''Babesia'' |
| name = ''Babesia'' |
||
| image = Babiesa spp.jpg |
| image = Babiesa spp.jpg |
||
| regnum = [[ |
| regnum = [[Eukaryota]] |
||
| phylum = [[ |
| phylum = [[Alveolata]] |
||
| classis = [[ |
| classis = [[Apicomplexa]] |
||
| ordo = [[ |
| ordo = [[Aconoidasida]] |
||
| familia = [[ |
| familia = [[Piroplasmida]] |
||
| genus = ''''' |
| genus = '''''Babesiidae''''' |
||
| subdivision_ranks = Species |
| subdivision_ranks = Species |
||
| subdivision = |
| subdivision = |
||
''[[Babesia bigemina]]''< |
''[[Babesia bigemina]]''< |
||
''[[Babesia bovis]]''<br /> |
''[[Babesia bovis]]''<br /> |
||
''[[Babesia canis]]''<br /> |
''[[Babesia canis]]''<br /> |
||
| Line 21: | Line 21: | ||
''[[Babesia jakimovi]]''<br /> |
''[[Babesia jakimovi]]''<br /> |
||
''[[Babesia major]]''<br /> |
''[[Babesia major]]''<br /> |
||
''Babesia microti'' |
''Babesia microti'' <br /> |
||
''[[Babesia ovate]]''<br /> |
''[[Babesia ovate]]''<br /> |
||
''[[Babesia pantherae]]'' |
''[[Babesia pantherae]]'' |
||
}} |
}} |
||
Babesia is a protozoan parasite of the blood that causes a hemolytic disease known as Babesiosis. There are over 100 species of Babesia identified however only a handful of species have been documented as pathogenic in humans [8]. In the United States, Babesia microti is the most common strain associated with humans with other species infecting cattle, livestock and occasionally domestic animals [1][2]. People who contract Babesiosis suffer from malaria-like symptoms. As a result malaria is a common misdiagnosis for the disease. |
|||
'''Babesia''' is a piroplasmid [[protozoa]]l [[parasite]] of blood cells and is found in the phylum [[Apicomplexa]]. This group is characterized by its round, rod or abstract shape and lack of any mobility structures such as [[cilia]] or [[flagella]]. The species ''B. bigemina'' was noted as the first vector-based disease described in history.<ref name="Babesiosis"> ''Babesiosis''. Center for Disease Control; Parasites and Health. Available at: http://www.dpd.cdc.gov/dpdx/HTML/Babesiosis.htm</ref> |
|||
== Classificaton and Taxonomy == |
|||
There are many different ''Babesia'' species, several of which can infect humans. Babesia is a parasite, which means that it lives at the expense of its host.<ref name="Roberts">Roberts, L.S. and J. Janovy, Jr. Gerald D. Schmidt & Larry S. Roberts’ Foundations of Parasitology, 7th edition, Boston: McGraw-Hill, 2005. pp 166-170.</ref> [[Babesiosis]], the disease caused by infection with ''Babesia'', is most common in [[New York]] (specifically, [[Long Island]]), [[Martha's Vineyard]], and [[Nantucket]], but fatalities are rare in humans.<ref name="Cuhna">Cuhna, Burke A., M.D. Babesiosis. Available at: http://www.emedicine.com/med/topic195.htm</ref> |
|||
Babesia is a protozoan parasite with a taxonomic classification as seen in Table 1. Babesia microti (B. microti) and Babesia divergens (B. divergens) are the two species to most frequently infect humans. Infections from other species of Babesia have been documented in humans but are not habitually seen. |
|||
== Zoonotic Species of Babesia == |
|||
Babesiosis can also be known as Prioplasmosis [1]. Due to historical misclassifications, the protozoa was labeled with many names that are no longer used. Common names of the disease include Texas Cattle Fever, Redwater Fever, Tick Fever, and Nantucket Fever [2]. |
|||
{|- |
|||
Below are the different types of Babesia that may be infective to humans. They are found in many parts of the world and are normally transmitted to humans through [[ticks]]. The ticks are a biologic [[vector (biology)|vector]] because part of the life cycle of the parasite takes place within the tick. ''B. bigemina'' affects cows and is one of the most common forms.<ref name="Roberts" /> |
|||
! Table 1 [3][4] |
|||
! |
|||
{| class="wikitable" |
|||
|- |
|- |
||
| Kindgom |
|||
! '''Species''' |
|||
| Eukaryota |
|||
! '''Host''' |
|||
! '''Vector''' |
|||
! '''Distribution''' |
|||
|- |
|- |
||
| Phylum |
|||
| ''B. divergens'' |
|||
| Alveolata |
|||
| Man |
|||
| Ticks |
|||
| Yugoslavia, Russia, Ireland, Scotland |
|||
|- |
|- |
||
| Class |
|||
| ''B. bigemina'' |
|||
| Apicomplexa |
|||
| Man |
|||
| Ticks |
|||
| Subtropics and Tropics |
|||
|- |
|- |
||
| Order |
|||
| ''B. equi'' |
|||
| Aconoidasida |
|||
| Man |
|||
| Ticks |
|||
| South America |
|||
|- |
|- |
||
| Family |
|||
| ''B. microfti'' |
|||
| Piroplasmida |
|||
| Man |
|||
| Ticks |
|||
| North America (Eastern US, Wisconsin), Asia, Russia, India, Africa |
|||
|- |
|- |
||
| Genus |
|||
| ''B. duncani'' |
|||
| Babesiidae |
|||
| Man |
|||
|- |
|||
| Ticks |
|||
| Species |
|||
| North America (Western US) Asia |
|||
| Over 100 species; Babesia microti; Babesia divergens |
|||
|} |
|} |
||
== |
== History == |
||
For centuries, Babesiosis was known to be a serious illness for wild and domestic animals especially cattle. Victor Babes, a Romanian scientist who first documented the disease in 1888, described symptoms of a severe hemolytic illness seen uniquely in cattle and sheep [2].Some years later, Americans Theobald Smith and Fred Kilborne identified the parasite as the cause of Texas Cattle Fever, the same disease described by Babes. Smith and Kilborne also identified the tick as the agent of transmission, a discovery that first introduced the concept of arthropods functioning as disease vectors [5]. Long believed to be a disease that only affected non-human mammals, it wasn’t until 1957 that the first case of Babesiosis was seen in humans [1]. The first case was observed in a splenectomized patient as were all people diagnosed up until 1969. The first case of Babesiosis seen in a non-splenectomized patient proved that the protozoan parasite was pathogenic to all people [6]. |
|||
=== History === |
|||
== Clinical Presentation == |
|||
This disease is responsible for Texas cattle fever (also called red-water fever) and was to blame for severe losses in the cattle production industry in the 1880s. After this fiasco, much more time and research was put into studying this disease and in the late 1880s, [[Theobald Smith]] and F.L. Kilbourne discovered the cause. The tick "boophilus" was found to be a vector. This was the first description of a vector-based disease on record. This discovery then lead to the effective prevention method of controlling the tick population. This disease has been eradicated in the United States, but is endemic in Mexico and Central America. Farmers in this country are taking many precautions to prevent the disease from entering the United States again.<ref name="Roberts" /> |
|||
The severity of B. microti infections varies. For 25% of cases in adults and half of cases in children, the disease is asymptomatic or mild with flu-like symptoms. In cases of symptomatic infection, symptoms are characterized by irregular fevers, chills, headaches, general lethargy, pain and malaise [1]. In severe cases, hemolytic anemia, jaundice, shortness of breath, and hemoglobinuria are documented due to the lytic effects of parasitic multiplication [13][2]. Immunocompetent individuals with healthy spleens often recover without treatment [1]. Splenectomized patients are more susceptible to contracting the disease and the course of infection often ends fatally within 5 to 8 days of symptom onset [7]. Parasitemia levels can reach up to 85% in patients without spleens compared to 1-10% in individuals with spleens and effective immune systems. Splenectomized patients suffer from severe hemolytic anemia with occasional incidences of hepatomegaly and splenomegaly documented [13]. |
|||
=== Life Cycle === |
|||
Complications that arise from B. microti infections include acute respiratory failure, congestive heart failure, and renal failure. Infections can be fatal in 5-10% of hospitalized patients with increased risk of death in the immunosupressed, the elderly, and those co-infected with Lyme disease [13]. |
|||
The infective stage of this parasite is termed the [[sporozoite]] and is found in the salivary gland of the tick. The sporozoite enters the vertebrate via tick bite.<ref name="Roberts" /> |
|||
B. divergens infections have a much higher fatality rate (42%) and present with the most severe symptoms. Infected individuals suffer from hemoglobinuria followed by jaundice, a persistently high fever, chills and sweats. If left untreated, B. divergens infections can develop into shock-like symptoms with pulmonary edema and renal failure [13]. |
|||
Signs of infection usually arise 1 to 8 weeks after a bite from an infectious tick [7]. Infections from B. divergens have a shorter latent period usually ranging from 1-3 weeks [13]. |
|||
==== Vertebrate Host Cycle ==== |
|||
== Transmission == |
|||
Once the sporozoite enters the human (or any other vertebrate), it rapidly infects the red blood cells. In the red blood cells, these sporozoites mature to the [[trophozoite]] stage and then rapidly undergo the process of merogany (a type of multiple fission where the parasite reproduces asexually) to produce [[merozoites]]. These merozoites then burst out of the red blood cells and infect other red blood cells and continue to multiply. However, some merozoites stay in the red blood cells and wait for the next host. This next host is usually a tick that is infected when biting the animal.<ref name="Roberts" /> |
|||
Babesia is spread through the saliva of a tick when it bites. At its nymphal stage, a tick will bite into the skin for a blood meal. The tick, if not removed, will stay attached for 3 to 6 days with longer periods of feeding associated with a higher probability of acquiring the parasite. The parasite can survive in the tick as it molts through its various developmental stages resulting in all stages being potentially infectious. Some species of Babesia can be transmitted from a female tick to its offspring before migrating to salivary glands for feeding [1]. B. microti, the most common variety of Babesia in humans however, has not been shown to transmit transovarialy [8]. |
|||
==== Biological Vector Host Cycle ==== |
|||
In the Americas, Ixodidae scapularis is the most common vector. This hard tick, commonly known as a deer tick, is also the vector for other tick-associated illnesses such as Lyme disease. Many species of Babesia only infect non-human mammalian hosts, most commonly cattle, horses, and sheep. B. microti and B. divergens are the two main pathogenic species in humans. Their reservoirs are theorized to be the white-footed mouse (Peromuscus Leucopus Rafinesque), the microtus vole (Microtus spp.), and the white-tailed deer (Odocoileus virginianus) [10]. These woodland species are hypothesized reservoirs because although they are known to harbor the disease, complete reservoir competence has not yet been shown [11]. |
|||
Inside of a single tick, the merozoites contracted from the blood of the infected vertebrate transform into ray bodies, which fuse to form the [[zygote]], which is called the primary kinete. However, in order for a vertebrate host to be infected, the parasite must infect the oocyte of the tick via [[transplacental]] transmission, which is accomplished when the primary kinetes leave the intestine of the tick and migrate to other cells, including those of the ovary. These primary kinetes then grow and multiply, producing [[cytomere|cytomeres]]. These cytomeres then become new, differentiated kinetes. Some of the kinetes travel to the salivary gland and cause enlargement of the host cells. The kinetes then divide rapidly while the tick is feeding to produce a very large number of sporozoites. The infected tick may then bite a vertebrate animal (including a human), thus rendering them infected.<ref name="Roberts" /> |
|||
Most cases of transmission between humans are attributed to a tick vector. However, as of 2003 the Centers for Disease Control and Prevention (CDC) acknowledged more than 40 cases of Babesiosis contracted from packed red blood cell (PRBC) transfusions and 2 infections documented from organ transplantation. PRBC transfusions that cause infections were identified through testing of the blood donor for B. microti antibodies [9]. The occurrence of PRBC transfusions as a mechanism of Babesia transmission puts pressure on governmental organizations, such as the CDC, to heighten standard measures for screening blood donations. |
|||
== Morphology == |
|||
Babesia enters erythrocytes at the sporozoite stage. Within the red blood cell, the protozoa become cyclical and develop into a trophozoite ring. The trophozoites morph into merozoites, which have a tetrad structure coined a Maltese-cross form [14]. The tetrad morphology, which can be seen with Geimsa staining of a thin blood smear, is unique to Babesia and serves as a distinguishing feature from Plasmodium falciparum, a protozoan of similar morphology that causes Malaria. Trophozoite and merozoite growth ruptures the host erythrocyte leading to the release of vermicules, the infectious parasitic bodies, which rapidly spread the protozoa throughout the blood [1]. |
|||
== Life Cycle == |
|||
The life cycle of B. microti requires a biological stage in a rodent or deer host. To begin, the Ixodidae introduces the sporozoites into the rodent when taking a blood meal. Sporozoites enter erythrocytes in the blood and begin the cyclical development between trophozoites and merozoites. Rather than producing more trophozoites, some merozoites produce gametocytes. The definitive tick host, Ixodidae, takes up the gametocytes when attached for a blood meal. The gametes are fertilized in the gut of the tick and develop into sporozoites in the salivary glands. The sporozoites are introduced into a human upon inoculation at the bite of an infected tick. Even as an incidental host, the phase changes that occur in the parasite are the same within humans as in the biological hosts. Babesia can be diagnosed at the trophozoite stage and can be transmitted from human to human either through the tick vector or through blood transfusions [8]. |
|||
[[Image:Babesia life cycle human en.svg|thumb|center|500px|Life Cycle of Babesia]] |
[[Image:Babesia life cycle human en.svg|thumb|center|500px|Life Cycle of Babesia]] |
||
== Diagnosis and Treatment == |
|||
=== Disease Pathogenesis of Babesia (Usually in Cows or Humans) === |
|||
{{main|Babesiosis}} |
|||
==== Clinical Signs ==== |
|||
=== Diagnostic Tests === |
|||
The temperature of the animal will rise rapidly, with a fever which persists for weeks. Dullness and listlessness with a loss of appetite are also common. [[Anemia]] due to loss of red blood cells becomes very severe. Also, because these red blood cells are being lysed (bursting), [[jaundice]] (a yellowing of the skin and eyes) and [[hemoglobinuria]] are common. Urine may appear red and internal organs may become damaged.<ref name="Roberts" /> |
|||
As a protozoan parasite, the most effective way to identify Babesia infection though blood sample testing. It is important to pay specific attention to particular morphologies of Babesia in blood smears because its substantial similarity to Malaria Plasmodium falciparum results in many patients suffering from Babesiosis being misdiagnosed. The few distinguishing factors for Babesia include protozoa with varying shapes and sizes, the potential to contain vacuoles, and the lack of pigment production. Trophozoites within an erythrocyte that appear in a tetrad formation are also indicative of Babesia. A trained eye is necessary to distinguish the two species. |
|||
==== Treatment ==== |
|||
Even with much study of Babesiosis and Malaria, misdiagnosis with blood smear can be frequent and problematic. To supplement a blood smear, diagnoses should be made with an indirect fluorescent antibody (IFA) test. IFA testing has a much higher specificity than stained blood smears with antibody detection in 88-96 % of infected patients [8]. Diagnostic measures through antibody testing are also particularly useful for identifying serum prevalence in asymptomatic individuals. Due to the transmissibility of Babesia through blood transfusions, IFA testing would be an effective means of screening for the disease in blood donations. |
|||
There are several types of drugs that may help to treat a babesia infection. The most common are [[clindamycin]] and [[atovaquone]].<ref name="Babesiosis" /> However, these drugs may have extreme side effects and are not usually used in the United States. If they are used in food source animals, they may leave residues in the food that people ingest, which is a health concern.<ref name="Roberts" /> |
|||
Historically, Babesiosis diagnosis was carried out with xenodiagnosis in hamsters for B. microti and in gerbils for B.divergens [1]. While successful at identifying the disease, this diagnostic technique has been abandoned for faster diagnostic measures. |
|||
==== Prevention ==== |
|||
=== Treatment === |
|||
There are several ways to manage and treat Babesiosis. In many cases, patients spontaneously recover having only experienced mild symptoms undiagnosed as the disease. This occurrence is almost always seen in B. microti infections, which are generally more common in the United States. For B. divergens and more severe B. microti infections, the standard treatment historically for symptomatic individuals was oral or intravenous Clindamycin with oral quinine [8]. With the results of research completed in 2000 however, treatment regimens have been increasingly leaning towards oral Atovaquone with oral azithromycin. The latter medications are preferred as they are equally effective and exhibit fewer associated adverse reactions [12]. In severe cases, blood exchange transfusions have been performed to lower the parasitic load in the individual [1]. Other rudimentary treatment measures include addressing and correcting abnormal clinical signals [2]. |
|||
== Epidemiology == |
|||
Of the species to infect humans, B. microti is most common in the Americas whereas B. divergens is the predominant strain found in Europe. Endemic areas are regions of tick habitat, including the forest regions of the Northeastern United States and temperate regions of Europe [7]. Ixodidae, the tick vector of B. microti, also transmits the better-known Lyme disease. For reasons that remain unclear, in areas endemic to both Lyme disease and Babesiosis, Lyme disease transmission prevails and is more predominant in the region [1]. Prevalence of Babesiosis is regions endemic to Malaria remains unknown due to the likelihood of misdiagnosis as Malaria [13]. As the disease results in a high number of asympomatic individuals, many populations can possess high seroprevalence without much documentation of illness. For example, in Rhode Island and Nantucket, seroprevalence has been measured to be 20-25% [1]. Prevalence of Babesiosis is most documented during the months of May to September where there is high tick activity in endemic regions [7]. |
|||
== Prevention == |
|||
The most effective public health measure for Babesia is avoidance of tick exposure. This can be performed through personal prevention strategies such as avoiding tick infested areas (especially during high tick season between May and September), remaining covered with light clothing, searching for ticks after being outdoors and removing discovered ticks from the skin [13]. Other preventative measures include applying Diethyltoluamide (DEET), a common bug repellent that is effective against ticks amongst other insects. On a state level, if health departments are particularly motivated, tick elimination is a possibility. In 1906, efforts were made to eradicate the tick vector of the bovine disease form of Babesiosis in the United States. Eradication was successfully completed four decades later [2]. Eradication efforts would be a long-term project, which would significantly reduce the prevalence of both Babesiosis and Lyme disease. However, as public health departments are often short on funding, preventative measures seem to be more recommended over vector control. Due to the relatively low prevalence of the disease and the presence of several reservoirs, Babesiosis is currently not a candidate for vaccine prevention. |
|||
== Useful Links == |
|||
Lyme and Tick-Borne Diseases Research Center: Babesiosis: [http://www.columbia-lyme.org/patients/tbd_babesia.html] |
|||
Connecticut Department of Public Health: Babesiosis Fact Sheet: [http://www.ct.gov/dph/cwp/view.asp?a=3136&q=388254] |
|||
New York State Department of Health: Babesiosis: [http://www.health.state.ny.us/diseases/communicable/babesiosis/fact_sheet.htm] |
|||
Centers for Disease Control and Prevention: [http://www.cdc.gov/ncidod/dpd/parasites/babesia/default.htm] |
|||
DPDx: Laboratory Identification of Parasites of Public Health Concern: Babesiosis: [http://www.dpd.cdc.gov/dpdx/HTML/Babesiosis.htm] |
|||
In non-human animals, the best prevention is dipping to kill off and prevent infestation of ticks on the animal. This helps to ensure that the animals do not contract any parasites from the tick in the first place.<ref name="Roberts" /> [[animal sentinels|Sentinels]] are animals that may give early warning of infection of a certain disease, which can, in turn, be used to prevent the spread of that disease in humans.<ref>Sentinels of Health Threats from Recreational Waters. United States Geological Survey. Available at: http://health.usgs.gov/sentinels/recreational_waters.html</ref> Dogs, cows and rats are all important sentinels of babesia.<ref>Quick, R.E., B.L. Herwald, J.W. Thomford, M.E. Garnett, M.L. Eberhard, M. Wilson, D.H. Spach, J.W. Dickerson, S.R.Telford 3rd, K.R. Steingart, R. Pollock, D.H. Persing, J.M. Kobayashi, D.D. Juranek, P.A. Conrad. Babesiosis in Washington State: a new species of Babesia? Available at: http://canarydatabase.org/record/560</ref> |
|||
==See also== |
==See also== |
||
*[[Babesiosis]] |
|||
*[[List of parasites (human)]] |
|||
== References == |
== References == |
||
{{Reflist}} |
|||
[1] Despommier, Dickson D. et al. Parasitic Diseases. Ed 3. Spinger-Verlag Inc: New York City, New York, 1995, p 224-226 |
|||
[2] M. Ristic et al, Ed. Malaria and Babesiosis: New Perspectives in Clinical Microbiology. Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1984, 100-170 |
|||
[3] Abeer Khayat and Mobeen Rathore. The Neurological Manifestations of Pediatric Infectious Diseases and Immunodeficiency Syndromes. Humana Press, 2008, Chapter 36, 343-346 |
|||
[4] National Center for Biotechnology Information: Taxonomy Browser. [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=5864&lvl=3&lin=f&keep=1&srchmode=1&unlock] |
|||
[5] Centers for Disease Control and Prevention. Theobald Smith. [http://www.cdc.gov/eid/content/14/12/1939.htm] |
|||
[6] Beaver, PhD., Sc.D., Paul Chester, et al. Clinical Parasitology. Ed 9. Lea and Febiger: Philadelphia, Pennsylvania, p 205-208 |
|||
[7] National Institute of Allergy and Infectious Diseases, National Institutes of Health. Babesiosis. [http://www3.niaid.nih.gov/topics/babesiosis/] |
|||
[8] DPDx: Laboratory Identification of Parasites of Public Health Concern. Babesiosis. [http://www.dpd.cdc.gov/dpdx/HTML/Babesiosis.htm] |
|||
[9] Joseph Z. Lux, Don Weiss, Jeanne V. Linden, Debra Kessler, Barbara L. Herwaldt, Susan J. Wong, Jan Keithly, Phyllis Della-Latta, and Brian E. Scully. Transfusion-Associated Babesiosis Infection after Heart Transplant. Emerging Infectious Diseases, Vol. 9, No. 2, 2003. [http://www.cdc.gov/ncidod/EID/vol9no1/02-0149.htm] |
|||
[10] G Karbowiak. Zoonotic reservoir of Babesia microti in Poland. Polish Journal of Microbiology, 2004. [http://www.ncbi.nlm.nih.gov/pubmed/15787199?dopt=Abstract] |
|||
[11] CAT.INST: Centre Nationale de Recherche Scientifique [http://cat.inist.fr/?aModele=afficheN&cpsidt=4605007] |
|||
[12] Peter J. Krause, M.D., Timothy Lepore, M.D., Vijay K. Sikand, M.D., Joseph Gadbaw, M.D., Georgine Burke, Ph.D., Sam R. Telford, Sc.D., Peter Brassard, M.D., Diane Pearl, M.D., Jaber Azlanzadeh, Ph.D., Diane Christianson, R.N., Debra McGrath, R.N., and Andrew Spielman, Sc.D. Atovaquone and Azithromycin for the Treatment of Babesiosis. New England Journal of Medicine, Vol 343: 1454-1458, 2000. [http://content.nejm.org/cgi/content/full/343/20/1454] |
|||
[13] Jeffrey A. Gelfand and Edouard Vannier. Chapter 204: Babesiosis. McGraw-Hill’s Access Medicine. [http://www.accessmedicine.com/content.aspx?aID=2892931] |
|||
[14] Barbara L. Herwaldt, Simone Cacciò, Filippo Gherlinzoni, Horst Aspöck, Susan B. Slemenda, PierPaolo Piccaluga, Giovanni Martinelli, Renate Edelhofer, Ursula Hollenstein, Giovanni Poletti, Silvio Pampiglione, Karin Löschenberger, Sante Tura, and Norman J. Pieniazek. Molecular Characterization of a Non–Babesia divergens Organism Causing Zoonotic Babesiosis in Europe. Emerging Infectious Diseases, Vol 9:8, 2003 |
|||
[http://www.cdc.gov/ncidod/EID/vol9no8/02-0748.htm#Figure2] |
|||
[[Category:Parasites]] |
[[Category:Parasites]] |
||
| Line 119: | Line 169: | ||
[[pt:Babesia]] |
[[pt:Babesia]] |
||
[[ru:Бабезия]] |
[[ru:Бабезия]] |
||
[[tk:Babeziýa]] |
|||
[[zh:巴倍虫属]] |
[[zh:巴倍虫属]] |
||
Revision as of 18:35, 26 February 2009
| Babesia | |
|---|---|
| |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Babesiidae
|
| Species | |
|
Babesia bigemina<
Babesia bovis | |
Babesia is a protozoan parasite of the blood that causes a hemolytic disease known as Babesiosis. There are over 100 species of Babesia identified however only a handful of species have been documented as pathogenic in humans [8]. In the United States, Babesia microti is the most common strain associated with humans with other species infecting cattle, livestock and occasionally domestic animals [1][2]. People who contract Babesiosis suffer from malaria-like symptoms. As a result malaria is a common misdiagnosis for the disease.
Classificaton and Taxonomy
Babesia is a protozoan parasite with a taxonomic classification as seen in Table 1. Babesia microti (B. microti) and Babesia divergens (B. divergens) are the two species to most frequently infect humans. Infections from other species of Babesia have been documented in humans but are not habitually seen. Babesiosis can also be known as Prioplasmosis [1]. Due to historical misclassifications, the protozoa was labeled with many names that are no longer used. Common names of the disease include Texas Cattle Fever, Redwater Fever, Tick Fever, and Nantucket Fever [2].
| Table 1 [3][4] | |
|---|---|
| Kindgom | Eukaryota |
| Phylum | Alveolata |
| Class | Apicomplexa |
| Order | Aconoidasida |
| Family | Piroplasmida |
| Genus | Babesiidae |
| Species | Over 100 species; Babesia microti; Babesia divergens |
History
For centuries, Babesiosis was known to be a serious illness for wild and domestic animals especially cattle. Victor Babes, a Romanian scientist who first documented the disease in 1888, described symptoms of a severe hemolytic illness seen uniquely in cattle and sheep [2].Some years later, Americans Theobald Smith and Fred Kilborne identified the parasite as the cause of Texas Cattle Fever, the same disease described by Babes. Smith and Kilborne also identified the tick as the agent of transmission, a discovery that first introduced the concept of arthropods functioning as disease vectors [5]. Long believed to be a disease that only affected non-human mammals, it wasn’t until 1957 that the first case of Babesiosis was seen in humans [1]. The first case was observed in a splenectomized patient as were all people diagnosed up until 1969. The first case of Babesiosis seen in a non-splenectomized patient proved that the protozoan parasite was pathogenic to all people [6].
Clinical Presentation
The severity of B. microti infections varies. For 25% of cases in adults and half of cases in children, the disease is asymptomatic or mild with flu-like symptoms. In cases of symptomatic infection, symptoms are characterized by irregular fevers, chills, headaches, general lethargy, pain and malaise [1]. In severe cases, hemolytic anemia, jaundice, shortness of breath, and hemoglobinuria are documented due to the lytic effects of parasitic multiplication [13][2]. Immunocompetent individuals with healthy spleens often recover without treatment [1]. Splenectomized patients are more susceptible to contracting the disease and the course of infection often ends fatally within 5 to 8 days of symptom onset [7]. Parasitemia levels can reach up to 85% in patients without spleens compared to 1-10% in individuals with spleens and effective immune systems. Splenectomized patients suffer from severe hemolytic anemia with occasional incidences of hepatomegaly and splenomegaly documented [13].
Complications that arise from B. microti infections include acute respiratory failure, congestive heart failure, and renal failure. Infections can be fatal in 5-10% of hospitalized patients with increased risk of death in the immunosupressed, the elderly, and those co-infected with Lyme disease [13]. B. divergens infections have a much higher fatality rate (42%) and present with the most severe symptoms. Infected individuals suffer from hemoglobinuria followed by jaundice, a persistently high fever, chills and sweats. If left untreated, B. divergens infections can develop into shock-like symptoms with pulmonary edema and renal failure [13].
Signs of infection usually arise 1 to 8 weeks after a bite from an infectious tick [7]. Infections from B. divergens have a shorter latent period usually ranging from 1-3 weeks [13].
Transmission
Babesia is spread through the saliva of a tick when it bites. At its nymphal stage, a tick will bite into the skin for a blood meal. The tick, if not removed, will stay attached for 3 to 6 days with longer periods of feeding associated with a higher probability of acquiring the parasite. The parasite can survive in the tick as it molts through its various developmental stages resulting in all stages being potentially infectious. Some species of Babesia can be transmitted from a female tick to its offspring before migrating to salivary glands for feeding [1]. B. microti, the most common variety of Babesia in humans however, has not been shown to transmit transovarialy [8].
In the Americas, Ixodidae scapularis is the most common vector. This hard tick, commonly known as a deer tick, is also the vector for other tick-associated illnesses such as Lyme disease. Many species of Babesia only infect non-human mammalian hosts, most commonly cattle, horses, and sheep. B. microti and B. divergens are the two main pathogenic species in humans. Their reservoirs are theorized to be the white-footed mouse (Peromuscus Leucopus Rafinesque), the microtus vole (Microtus spp.), and the white-tailed deer (Odocoileus virginianus) [10]. These woodland species are hypothesized reservoirs because although they are known to harbor the disease, complete reservoir competence has not yet been shown [11].
Most cases of transmission between humans are attributed to a tick vector. However, as of 2003 the Centers for Disease Control and Prevention (CDC) acknowledged more than 40 cases of Babesiosis contracted from packed red blood cell (PRBC) transfusions and 2 infections documented from organ transplantation. PRBC transfusions that cause infections were identified through testing of the blood donor for B. microti antibodies [9]. The occurrence of PRBC transfusions as a mechanism of Babesia transmission puts pressure on governmental organizations, such as the CDC, to heighten standard measures for screening blood donations.
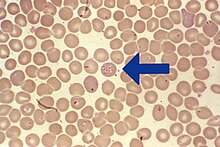
Morphology
Babesia enters erythrocytes at the sporozoite stage. Within the red blood cell, the protozoa become cyclical and develop into a trophozoite ring. The trophozoites morph into merozoites, which have a tetrad structure coined a Maltese-cross form [14]. The tetrad morphology, which can be seen with Geimsa staining of a thin blood smear, is unique to Babesia and serves as a distinguishing feature from Plasmodium falciparum, a protozoan of similar morphology that causes Malaria. Trophozoite and merozoite growth ruptures the host erythrocyte leading to the release of vermicules, the infectious parasitic bodies, which rapidly spread the protozoa throughout the blood [1].
Life Cycle
The life cycle of B. microti requires a biological stage in a rodent or deer host. To begin, the Ixodidae introduces the sporozoites into the rodent when taking a blood meal. Sporozoites enter erythrocytes in the blood and begin the cyclical development between trophozoites and merozoites. Rather than producing more trophozoites, some merozoites produce gametocytes. The definitive tick host, Ixodidae, takes up the gametocytes when attached for a blood meal. The gametes are fertilized in the gut of the tick and develop into sporozoites in the salivary glands. The sporozoites are introduced into a human upon inoculation at the bite of an infected tick. Even as an incidental host, the phase changes that occur in the parasite are the same within humans as in the biological hosts. Babesia can be diagnosed at the trophozoite stage and can be transmitted from human to human either through the tick vector or through blood transfusions [8].

Diagnosis and Treatment
Diagnostic Tests
As a protozoan parasite, the most effective way to identify Babesia infection though blood sample testing. It is important to pay specific attention to particular morphologies of Babesia in blood smears because its substantial similarity to Malaria Plasmodium falciparum results in many patients suffering from Babesiosis being misdiagnosed. The few distinguishing factors for Babesia include protozoa with varying shapes and sizes, the potential to contain vacuoles, and the lack of pigment production. Trophozoites within an erythrocyte that appear in a tetrad formation are also indicative of Babesia. A trained eye is necessary to distinguish the two species.
Even with much study of Babesiosis and Malaria, misdiagnosis with blood smear can be frequent and problematic. To supplement a blood smear, diagnoses should be made with an indirect fluorescent antibody (IFA) test. IFA testing has a much higher specificity than stained blood smears with antibody detection in 88-96 % of infected patients [8]. Diagnostic measures through antibody testing are also particularly useful for identifying serum prevalence in asymptomatic individuals. Due to the transmissibility of Babesia through blood transfusions, IFA testing would be an effective means of screening for the disease in blood donations.
Historically, Babesiosis diagnosis was carried out with xenodiagnosis in hamsters for B. microti and in gerbils for B.divergens [1]. While successful at identifying the disease, this diagnostic technique has been abandoned for faster diagnostic measures.
Treatment
There are several ways to manage and treat Babesiosis. In many cases, patients spontaneously recover having only experienced mild symptoms undiagnosed as the disease. This occurrence is almost always seen in B. microti infections, which are generally more common in the United States. For B. divergens and more severe B. microti infections, the standard treatment historically for symptomatic individuals was oral or intravenous Clindamycin with oral quinine [8]. With the results of research completed in 2000 however, treatment regimens have been increasingly leaning towards oral Atovaquone with oral azithromycin. The latter medications are preferred as they are equally effective and exhibit fewer associated adverse reactions [12]. In severe cases, blood exchange transfusions have been performed to lower the parasitic load in the individual [1]. Other rudimentary treatment measures include addressing and correcting abnormal clinical signals [2].
Epidemiology
Of the species to infect humans, B. microti is most common in the Americas whereas B. divergens is the predominant strain found in Europe. Endemic areas are regions of tick habitat, including the forest regions of the Northeastern United States and temperate regions of Europe [7]. Ixodidae, the tick vector of B. microti, also transmits the better-known Lyme disease. For reasons that remain unclear, in areas endemic to both Lyme disease and Babesiosis, Lyme disease transmission prevails and is more predominant in the region [1]. Prevalence of Babesiosis is regions endemic to Malaria remains unknown due to the likelihood of misdiagnosis as Malaria [13]. As the disease results in a high number of asympomatic individuals, many populations can possess high seroprevalence without much documentation of illness. For example, in Rhode Island and Nantucket, seroprevalence has been measured to be 20-25% [1]. Prevalence of Babesiosis is most documented during the months of May to September where there is high tick activity in endemic regions [7].
Prevention
The most effective public health measure for Babesia is avoidance of tick exposure. This can be performed through personal prevention strategies such as avoiding tick infested areas (especially during high tick season between May and September), remaining covered with light clothing, searching for ticks after being outdoors and removing discovered ticks from the skin [13]. Other preventative measures include applying Diethyltoluamide (DEET), a common bug repellent that is effective against ticks amongst other insects. On a state level, if health departments are particularly motivated, tick elimination is a possibility. In 1906, efforts were made to eradicate the tick vector of the bovine disease form of Babesiosis in the United States. Eradication was successfully completed four decades later [2]. Eradication efforts would be a long-term project, which would significantly reduce the prevalence of both Babesiosis and Lyme disease. However, as public health departments are often short on funding, preventative measures seem to be more recommended over vector control. Due to the relatively low prevalence of the disease and the presence of several reservoirs, Babesiosis is currently not a candidate for vaccine prevention.
Useful Links
Lyme and Tick-Borne Diseases Research Center: Babesiosis: [1]
Connecticut Department of Public Health: Babesiosis Fact Sheet: [2]
New York State Department of Health: Babesiosis: [3]
Centers for Disease Control and Prevention: [4]
DPDx: Laboratory Identification of Parasites of Public Health Concern: Babesiosis: [5]
See also
References
[1] Despommier, Dickson D. et al. Parasitic Diseases. Ed 3. Spinger-Verlag Inc: New York City, New York, 1995, p 224-226
[2] M. Ristic et al, Ed. Malaria and Babesiosis: New Perspectives in Clinical Microbiology. Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1984, 100-170
[3] Abeer Khayat and Mobeen Rathore. The Neurological Manifestations of Pediatric Infectious Diseases and Immunodeficiency Syndromes. Humana Press, 2008, Chapter 36, 343-346
[4] National Center for Biotechnology Information: Taxonomy Browser. [6]
[5] Centers for Disease Control and Prevention. Theobald Smith. [7]
[6] Beaver, PhD., Sc.D., Paul Chester, et al. Clinical Parasitology. Ed 9. Lea and Febiger: Philadelphia, Pennsylvania, p 205-208
[7] National Institute of Allergy and Infectious Diseases, National Institutes of Health. Babesiosis. [8]
[8] DPDx: Laboratory Identification of Parasites of Public Health Concern. Babesiosis. [9]
[9] Joseph Z. Lux, Don Weiss, Jeanne V. Linden, Debra Kessler, Barbara L. Herwaldt, Susan J. Wong, Jan Keithly, Phyllis Della-Latta, and Brian E. Scully. Transfusion-Associated Babesiosis Infection after Heart Transplant. Emerging Infectious Diseases, Vol. 9, No. 2, 2003. [10]
[10] G Karbowiak. Zoonotic reservoir of Babesia microti in Poland. Polish Journal of Microbiology, 2004. [11]
[11] CAT.INST: Centre Nationale de Recherche Scientifique [12]
[12] Peter J. Krause, M.D., Timothy Lepore, M.D., Vijay K. Sikand, M.D., Joseph Gadbaw, M.D., Georgine Burke, Ph.D., Sam R. Telford, Sc.D., Peter Brassard, M.D., Diane Pearl, M.D., Jaber Azlanzadeh, Ph.D., Diane Christianson, R.N., Debra McGrath, R.N., and Andrew Spielman, Sc.D. Atovaquone and Azithromycin for the Treatment of Babesiosis. New England Journal of Medicine, Vol 343: 1454-1458, 2000. [13]
[13] Jeffrey A. Gelfand and Edouard Vannier. Chapter 204: Babesiosis. McGraw-Hill’s Access Medicine. [14]
[14] Barbara L. Herwaldt, Simone Cacciò, Filippo Gherlinzoni, Horst Aspöck, Susan B. Slemenda, PierPaolo Piccaluga, Giovanni Martinelli, Renate Edelhofer, Ursula Hollenstein, Giovanni Poletti, Silvio Pampiglione, Karin Löschenberger, Sante Tura, and Norman J. Pieniazek. Molecular Characterization of a Non–Babesia divergens Organism Causing Zoonotic Babesiosis in Europe. Emerging Infectious Diseases, Vol 9:8, 2003 [15]
