Iodopropynyl butylcarbamate: Difference between revisions
typo: Internation→International, new Sources section, format sources |
Una Blogga (talk | contribs) m Added language to balance the level of transparency on this article. |
||
| Line 47: | Line 47: | ||
IPBC Shows Very Low Sensitivity in Human Skin Tests: The Safety Assessment report [Lanigan, 1998] also included 32 studies between 1990 - 1994 in 3,582 subjects using skin application of IPBC at relevant concentrations. All 32 studies showed no skin sensitivity/irritation compared to placebo controls. In addition, two skin sensitivity studies on 183 children ages 3 – 12 yrs also showed no adverse effects as well as no significant irritation from IPBC. |
IPBC Shows Very Low Sensitivity in Human Skin Tests: The Safety Assessment report [Lanigan, 1998] also included 32 studies between 1990 - 1994 in 3,582 subjects using skin application of IPBC at relevant concentrations. All 32 studies showed no skin sensitivity/irritation compared to placebo controls. In addition, two skin sensitivity studies on 183 children ages 3 – 12 yrs also showed no adverse effects as well as no significant irritation from IPBC. |
||
Since the early safety report, there have been a few reports of human skin sensitivity to IPBC in individual patients – all of which showed complete recovery after discontinuance of use of any product containing the IPBC which was presumably an allergen for these patients [Toholka & Nixon, 2014; Pazzaglia & Tosti, 1999]. Post-1996 tests of human sensitivity to IPBC have all shown quite low sensitivity, having overall reported human skin testing (patch test) on 53,774 subjects with only 491 of those subjects showing any reaction (0.8%) to IPBC. In every study, positive patch test reactions occurred in less than 1% of subjects tested in all but one study. This is a very low reaction rate, but it is not zero, and the industry reports this low rate of reaction even though in the largest study of 25,435 subjects over 69% of the reactions were either weak or doubtful [Warshaw et al., 2013a]. These combined studies showing prevalence of reaction below 1% means that IPBC at this time does not have the reaction rates necessary to be included as an allergen in standard allergy series. But, it remains under close monitoring as it is a relatively new preservative for cosmetic products and will presumably increase in usage [Sasseville, 2004]. Most human patch tests performed before 2004 were with 0.1% IPBC solutions, i.e. 10 times the concentration used in many cosmetic products. Some used 0.5% IPBC. In 2004, it was suggested that a better concentration for tests of this substance would be 0.2% [Brasch et al., 2004] and this has contributed to the diagnosis of more sensitizations to this substance [Martin-Gorgojo & Johansen, 2013]. One study showed significantly increased sensitivity between 2005 and 2010 using 0.5% IPBC in patch tests [Warshaw et al., 2013b]. |
Since the early safety report, there have been a few reports of human skin sensitivity to IPBC in individual patients – all of which showed complete recovery after discontinuance of use of any product containing the IPBC which was presumably an allergen for these patients [Toholka & Nixon, 2014; Pazzaglia & Tosti, 1999]. Post-1996 tests of human sensitivity to IPBC have all shown quite low sensitivity, having overall reported human skin testing (patch test) on 53,774 subjects with only 491 of those subjects showing any reaction (0.8%) to IPBC. In every study, positive patch test reactions occurred in less than 1% of subjects tested in all but one study. This is a very low reaction rate, but it is not zero, and the industry reports this low rate of reaction even though in the largest study of 25,435 subjects over 69% of the reactions were either weak or doubtful [Warshaw et al., 2013a]. These combined studies showing prevalence of reaction below 1% means that IPBC at this time does not have the reaction rates necessary to be included as an allergen in standard allergy series. But, it remains under close monitoring as it is a relatively new preservative for cosmetic products and will presumably increase in usage [Sasseville, 2004]. Most human patch tests performed before 2004 were with 0.1% IPBC solutions, i.e. 10 times the concentration used in many cosmetic products. Some used 0.5% IPBC. In 2004, it was suggested that a better concentration for tests of this substance would be 0.2% [Brasch et al., 2004] and this has contributed to the diagnosis of more sensitizations to this substance [Martin-Gorgojo & Johansen, 2013]. One study showed significantly increased sensitivity between 2005 and 2010 using 0.5% IPBC in patch tests [Warshaw et al., 2013b]. |
||
UPDATE: Per EWG.org — Iodopropynyl Butylcarbamate does show "Limited evidence of gastrointestinal or liver toxicity" per the US EPA; that there is strong evidence of "Human toxicant or allergen" affects, by Cosmetic Ingredient Review Assessments; that it is "Suspected to be an environmental toxin" from Environment Canada Domestic Substance List, and that at least "50 studies in PubMed Science Library may include information on the toxicity of this chemical," NLM PubMed. <ref>https:// http://www.ewg.org/skindeep/ingredient/703111/IODOPROPYNYL_BUTYLCARBAMATE/</ref> |
|||
== See also == |
== See also == |
||
Revision as of 18:03, 25 May 2015
| |
| Names | |
|---|---|
| IUPAC name
3-Iodoprop-2-yn-1-yl butylcarbamate
| |
| Other names
3-Iodo-2-propynyl N-butylcarbamate; 3-Iodo-2-propynyl butylcarbamate; Iodocarb
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | IPBC |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.054.188 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H12INO2 | |
| Molar mass | 281.093 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
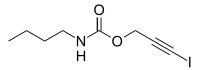
Iodopropynyl butylcarbamate (IPBC) is a water-based preservative agent originally used in the wood and paint industries, now also used in some cosmetics and personal care formulations. IPBC is a member of the carbamate family of biocides.[1]
Use is restricted in some countries due to its toxicity, especially acute inhalation toxicity. IPBC is also becoming recognized as a contact allergen.[2]
IPBC is an effective fungicide at very low concentrations in cosmetic and other products, and has shown very low sensitivity in humans tested with this preservative. IPBC was approved in 1996 for use up to 0.1% concentrations in topical products and cosmetics. However, this preservative is mostly found in cosmetics at about one-eighth that level [Maier et al., 2009]. IPBC Toxicity and Safety Tests show it to be generally safe: When used properly in leave-on skin products, IPBC is extremely safe [Steinberg, 2002]. Previous to being approved for cosmetic use in 1996, extensive safety and toxicity tests were conducted on IPBC and their results were gathered along with earlier studies in a report of the Safety Assessment of IPBC by the Cosmetic Ingredient Review [CIR Final Report, Lanigan 1998]. This final report found IPBC to be a non-carcinogen with no genotoxicity and in reproductive and developmental toxicity studies using rats and mice, IPBC had no significant effect on fertility, reproductive performance, or on the incidence of fetal malformation [Lanigan, 1998].
IPBC Shows Very Low Sensitivity in Human Skin Tests: The Safety Assessment report [Lanigan, 1998] also included 32 studies between 1990 - 1994 in 3,582 subjects using skin application of IPBC at relevant concentrations. All 32 studies showed no skin sensitivity/irritation compared to placebo controls. In addition, two skin sensitivity studies on 183 children ages 3 – 12 yrs also showed no adverse effects as well as no significant irritation from IPBC. Since the early safety report, there have been a few reports of human skin sensitivity to IPBC in individual patients – all of which showed complete recovery after discontinuance of use of any product containing the IPBC which was presumably an allergen for these patients [Toholka & Nixon, 2014; Pazzaglia & Tosti, 1999]. Post-1996 tests of human sensitivity to IPBC have all shown quite low sensitivity, having overall reported human skin testing (patch test) on 53,774 subjects with only 491 of those subjects showing any reaction (0.8%) to IPBC. In every study, positive patch test reactions occurred in less than 1% of subjects tested in all but one study. This is a very low reaction rate, but it is not zero, and the industry reports this low rate of reaction even though in the largest study of 25,435 subjects over 69% of the reactions were either weak or doubtful [Warshaw et al., 2013a]. These combined studies showing prevalence of reaction below 1% means that IPBC at this time does not have the reaction rates necessary to be included as an allergen in standard allergy series. But, it remains under close monitoring as it is a relatively new preservative for cosmetic products and will presumably increase in usage [Sasseville, 2004]. Most human patch tests performed before 2004 were with 0.1% IPBC solutions, i.e. 10 times the concentration used in many cosmetic products. Some used 0.5% IPBC. In 2004, it was suggested that a better concentration for tests of this substance would be 0.2% [Brasch et al., 2004] and this has contributed to the diagnosis of more sensitizations to this substance [Martin-Gorgojo & Johansen, 2013]. One study showed significantly increased sensitivity between 2005 and 2010 using 0.5% IPBC in patch tests [Warshaw et al., 2013b].
UPDATE: Per EWG.org — Iodopropynyl Butylcarbamate does show "Limited evidence of gastrointestinal or liver toxicity" per the US EPA; that there is strong evidence of "Human toxicant or allergen" affects, by Cosmetic Ingredient Review Assessments; that it is "Suspected to be an environmental toxin" from Environment Canada Domestic Substance List, and that at least "50 studies in PubMed Science Library may include information on the toxicity of this chemical," NLM PubMed. [3]
See also
References
- ^ "Chemical Book CAS No. 55406-53-6".
- ^ Badreshia, S; Marks Jr, JG (2002). "Iodopropynyl butylcarbamate". American journal of contact dermatitis. 13 (2): 77–9. PMID 12022126.
- ^ https:// http://www.ewg.org/skindeep/ingredient/703111/IODOPROPYNYL_BUTYLCARBAMATE/
Sources
- Brasch, J., et al. (2004). "Iodopropynylbutyl carbamate 0.2% is suggested for patch testing of patients with eczema possibly related to preservatives." Br J Dermatol 151(3): 608-615.
- Bryld, L. E., et al. (2001). "Allergic contact dermatitis from 3-iodo-2-propynyl-butylcarbamate (IPBC) - an update." Contact Dermatitis 44(5): 276-278.
- Bryld, L. E., et al. (1997). "Iodopropynyl butylcarbamate: a new contact allergen." Contact Dermatitis 36(3): 156-158.
- Lanigan, R. S. (1998). "Final Report On the Safety ASsessment of Iodopropynyl Butylcarbamate (IPBC)." International Journal of Toxicology 17(5 suppl): 1 - 37.
- Maier, L. E., et al. (2009). "Hand dermatitis: a focus on allergic contact dermatitis to biocides." Dermatol Clin 27(3): 251-264, v-vi.
- Martin-Gorgojo, A. and J. D. Johansen (2013). "Contact dermatitis caused by iodopropynyl butylcarbamate in Denmark." Contact Dermatitis 69(2): 78-85.
- Pazzaglia, M. and A. Tosti (1999). "Allergic contact dermatitis from 3-iodo-2-propynyl-butylcarbamate in a cosmetic cream." Contact Dermatitis 41(5): 290.
- Sasseville, D. (2004). "Hypersensitivity to preservatives." Dermatol Ther 17(3): 251-263.
- Schnuch, A., et al. (2002). "The preservative iodopropynyl butylcarbamate: frequency of allergic reactions and diagnostic considerations." Contact Dermatitis 46(3): 153-156.
- Steinberg, D. C. (2002). "Iodopropynyl butylcarbamate as a preservative." Am J Contact Dermat 13(4): 207-208.
- Thyssen, J. P., et al. (2010). "Temporal trends of preservative allergy in Denmark (1985-2008)." Contact Dermatitis 62(2): 102-108.
- Toholka, R. and R. Nixon (2014). "Suspected allergic contact dermatitis to iodopropynyl butylcarbamate in an alcohol hand rub commonly used in Australian health-care settings." Australas J Dermatol 55(1): 70-71.
- Warshaw, E. M., et al. (2008). "North American Contact Dermatitis Group patch-test results, 2003-2004 study period." Dermatitis 19(3): 129-136.
- Warshaw, E. M., et al. (2013a). "Positive patch test reactions to carba mix and iodopropynyl butylcarbamate: data from the North American Contact Dermatitis Group, 1998-2008." Dermatitis 24(5): 241-245.
- Warshaw, E. M., et al. (2013b). "North American Contact Dermatitis Group patch test results: 2009 to 2010." Dermatitis 24(2): 50-59.
External links
- "NA44:Iodopropynyl butylcarbamate" (PDF).
- Iodopropynyl butylcarbamate on the Skin Deep Cosmetics Database
