Noscapine: Difference between revisions
m Reference to possible cancer activity. |
Barnsoldat91 (talk | contribs) |
||
| Line 56: | Line 56: | ||
*[[Increased heart rate]] |
*[[Increased heart rate]] |
||
*[[Shaking]] and [[muscle spasms]] |
*[[Shaking]] and [[muscle spasms]] |
||
*Chest pains |
|||
*Increased alertness |
*Increased alertness |
||
*Loss of any sleepiness |
*Loss of any sleepiness |
||
Revision as of 22:23, 4 January 2009
This article may need to be rewritten to comply with Wikipedia's quality standards. |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Narcotine |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ~30% |
| Elimination half-life | 1.5 to 4h (mean 2.5) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.455 |
| Chemical and physical data | |
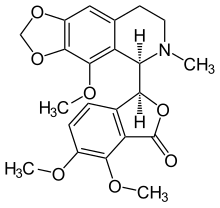
| Formula | C22H23NO7 |
| Molar mass | 413.421 g·mol−1 |
Noscapine (also known as Narcotine or Anarcotine) is a benzylisoquinoline alkaloid from plants of the Papaveraceae family, without significant painkilling properties. This agent is primarily used for its antitussive (cough-suppressing) effects. It has also been shown to have anticancer activity. [1]
Structure analysis
Naturally it occurs as the alpha enantiomer. It can be converted into the beta enantiomer when it is dissolved in alkaline water-ethanol solutions. The lactone ring is unstable and opens in basic media. The opposite reaction is presented in acidic media. The bond C1-C3' is also unstable. This is the bond connecting the two optically active carbon atoms. In aqueous solution of sulphuric acid and heating it dissociates into Cotarnine (4-methoxy- 6-methyl- 5,6,7,8-tetrahydro- [1,3]dioxolo [4,5-g]isoquinoline) and Opic acid (6-formyl- 2,3-dimethoxybenzoic acid). When Noscapine is reduced with Zn/HCl the bond C1-C3' saturates and the molecule dissociates into Hydrocotarnine (2-hydroxycotarnine) and Meconine (6,7-dimethoxyisobenzofuran -1(3H)-one).
Mechanism of action
Noscapine's antitussive effects appear to be primarily mediated by its sigma receptor agonist activity. Evidence for this mechanism is suggested by experimental evidence in rats. Pretreatment with rimcazole, a sigma specific antagonist, causes a dose-dependent reduction in antitussive activity of noscapine.[2]
Cancer and stroke treatment
Noscapine is currently under investigation for use in the treatment of several cancers and hypoxic ischemia in stroke patients. In cancer treatment, noscapine appears to interfere with microtubule function, and thus the division of cancer cells in a way similar to the taxanes. Early studies in treatment of prostate cancer are very promising.[3]
In stroke patients, noscapine blocks the bradykinine b-2 receptors. A 2003 study in Iran showed a dramatic decrease in mortality in patients treated with noscapine.[4]
Studies are currently underway to assess the effectiveness of this drug in cancer and stroke treatment. Noscapine is non-addictive, widely available, has a low side-effect incidence, and is easily administered orally, thus it has great potential for use, especially in developing countries.
Abuse
Noscapine (Nospen) has a history of Over-the-counter drug abuse in a several countries being readily available from local pharmacies as a prescription drug. The effects, beginning around 45 to 120 mins after consumption, are similar to dextromethorphan and alcohol intoxication. Abuse of noscapine and other cough suppressants (dextromethorphan, codeine, and antihistamines) has been reported to cause chronic cough lasting over one month upon withdrawal.[5] Unlike dextromethorphan, noscapine is not an NMDA receptor antagonist.[6]
Noscapine in Heroin
Noscapine can survive the manufacturing processes of heroin and can be found in street heroin. This is useful for law enforcement agencies, as the amounts of contaminants can identify the source of seized drugs. In 2005 in Liège, Belgium, the average noscapine concentration was around 8%.[7]
Noscapine has also been used to identify drug users who are taking street heroin at the same time as prescribed diamorphine.[8] Since the diamorphine in street heroin is the same as the pharmaceutical diamorphine, examination of the contaminants is the only way to test whether street heroin has been used. Other contaminants used in urine samples alongside noscapine include papaverine and acetylcodeine. Noscapine is metabolised by the body, and is itself rarely found in urine, instead being present as the primary metabolites, cotarnine and meconine. Detection is performed by gas chromatography-mass spectrometry or Liquid Chromatography-Mass Spectrometry (LCMS) but can also use a variety of other analytical techniques.
Possible side-effects
- Loss of coordination
- Hallucinations (auditory and visual)
- Loss of sexual drive
- Swelling of prostate
- Loss of appetite
- Dilated pupils
- Increased heart rate
- Shaking and muscle spasms
- Chest pains
- Increased alertness
- Loss of any sleepiness
- Loss of stereoscopic vision
The effects shown above are not permanent; the user may experience spasms the following day after yawning.
Noscapine should not be taken with any MAOIs (monoamine oxidase inhibitors), as unknown and potentially fatal effects may occur.
References
- ^ "Cold med ingredient may treat prostate cancer". MSNBC. Retrieved 2009-01-02.
- ^ Kamei J (1996). "Role of opioidergic and serotonergic mechanisms in cough and antitussives". Pulmonary pharmacology. 9 (5–6): 349–56. doi:10.1006/pulp.1996.0046. PMID 9232674.
- ^ "Noscapine effective against prostate cancer". Retrieved 2007-07-16.
- ^ "A preliminary report on the application of noscapine in the treatment of stroke". Retrieved 2007-07-16.
- ^ M.S. Bhatia and Lakshi Vaid (2005). "Pattern of drug abuse in patients with a psychogenic cough". Retrieved 2007-05-21.
- ^ PMID 2673498
- ^ Denooz R, Dubois N, Charlier C (2005). "[Analysis of two year heroin seizures in the Liege area]". Revue médicale de Liège (in French). 60 (9): 724–8. PMID 16265967.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Paterson S, Lintzeris N, Mitchell TB, Cordero R, Nestor L, Strang J (2005). "Validation of techniques to detect illicit heroin use in patients prescribed pharmaceutical heroin for the management of opioid dependence". Addiction. 100 (12): 1832–9. doi:10.1111/j.1360-0443.2005.01225.x. PMID 16367984.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
