Tris(8-hydroxyquinolinato)aluminium: Difference between revisions
Appearance
Content deleted Content added
further research reference |
|||
| Line 38: | Line 38: | ||
== See also == |
== See also == |
||
[[http://article.pubs.nrc-cnrc.gc.ca/ppv/RPViewDoc?issn=1480-3291&volume=49&issue=10&startPage=1683]] |
[[http://article.pubs.nrc-cnrc.gc.ca/ppv/RPViewDoc?issn=1480-3291&volume=49&issue=10&startPage=1683 "Kinetics of Reactions of 8-Quinolinol and Acetate with Hydroxyaluminum Species in Aqueous Solutions. 1. Polynuclear Hydroxyaluminum Cations1, R. C. TURNER AND WANS SULAIMAN Soil Research Institute, Research Branch, Canada Department of Agriculture, Ottawa, Canada, April 1970]] |
||
Revision as of 23:44, 7 March 2009
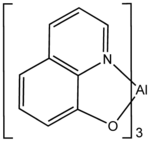
| |
| Names | |
|---|---|
| IUPAC name
Tris(8-hydroxyquinolinato)aluminium
| |
| Other names
8-Hydroxyquinoline aluminum salt, Alq3, Aluminum 8-hydroxyquinolinate, Aluminum oxinate,
| |
| Identifiers | |
| ECHA InfoCard | 100.016.570 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C27H18AlN3O3 | |
| Molar mass | 459.43 |
| Appearance | Yellow Powder |
| Melting point | >300 °C |
| insol in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tris(8-hydroxyquinolinato)aluminium is the chemical compound with the formula Al(C9H6NO)3. Widely abbreviated Alq3, it is a coordination complex wherein aluminium is bonded in a bidentate manner to the conjugate base of three 8-hydroxyquinoline ligands. Both the meriodonal and facial isomers are known as well as several polymorphs (different crystalline forms).[1] The compound is prepared by the reaction of 8-hydroxyquinoline with aluminium(III) sources[2]
- Al3+ + C9H6NOH → Al(C9H6NO)3 + 3 H+
Alq3 is a common component of organic light-emitting diodes (OLED's). Variations in the substituents on the quinoline rings affect its luminescence properties.[3]
References
- ^ Michael Cölle, Robert E. Dinnebier and Wolfgang Brütting "The structure of the blue luminescent -phase of tris(8-hydroxyquinoline)aluminium(III) (Alq3)" Chemical Communications 2002, pp. 2908-9. doi:10.1039/b209164j
- ^ Katakura, R.; Koide, Y. “Configuration-Specific Synthesis of the Facial and Meridional Isomers of Tris(8-hydroxyquinolinate)aluminum (Alq3)” Inorganic Chemistry 2006 volume 45,pp 5730-5732. doi:10.1021/ic060594s
- ^ Victor A. Montes, Radek Pohl, Joseph Shinar, Pavel Anzenbacher Jr. "Effective Manipulation of the Electronic Effects and Its Influence on the Emission of 5-Substituted Tris(8-quinolinolate) Aluminum(III) Complexes" Chemistry - A European Journal 2006, Volume 12, pp. p 4523-4535. doi:10.1002/chem.200501403
