Rydberg matter: Difference between revisions
No edit summary |
|||
| Line 19: | Line 19: | ||
[[Image:Decay schematic.jpg|right|thumb|290px|Schematic of an effective potential within a [[Wigner-Seitz cell]] of a Rydberg matter made of excited (n=10) Cs atoms.]] |
[[Image:Decay schematic.jpg|right|thumb|290px|Schematic of an effective potential within a [[Wigner-Seitz cell]] of a Rydberg matter made of excited (n=10) Cs atoms.]] |
||
Circular Rydberg states of atoms are extremely long-lived against deexcitation by emission of radiation. The so called radiative lifetime of a circular Rydberg state in n = 100 is approximately 1 second. This means that it decays with a characteristic lifetime of 1 second <ref>J. Liang, M. Gross, P. Goy, S. Haroche, "Circular Rydberg-state spectroscopy". Phys. Rev. A 33 (1986) 4437-4439. </ref>. The lifetime averaged over the angular momentum quantum numbers is 0.18 s at n = 40 and 17 s for n = 100 <ref>I. L. Beigman and V. S. Lebedev, "Collision theory of Rydberg atoms with neutral and charged particles". Phys. Rep. 250, 95 (1995).</ref>. The main reasons for such long lifetimes of atoms with excitation energy of several eV are the lack of spatial overlap between the excited circular orbital and low orbitals close to the atom, and the forbidden nature of transitions from high orbitals to low orbitals since the strong selection rule Δ''l'' = -1 is operative in a dipole transition. Similar effects exist in the condensed Rydberg matter: lack of orbital spatial overlap and angular momentum conservation as described make the lifetime of Rydberg matter long. |
Circular Rydberg states of atoms are extremely long-lived against deexcitation by emission of radiation. The so called radiative lifetime of a circular Rydberg state in n = 100 is approximately 1 second. This means that it decays with a characteristic lifetime of 1 second <ref>J. Liang, M. Gross, P. Goy, S. Haroche, "Circular Rydberg-state spectroscopy". Phys. Rev. A 33 (1986) 4437-4439. </ref>. The lifetime averaged over the angular momentum quantum numbers is 0.18 s at n = 40 and 17 s for n = 100 <ref>I. L. Beigman and V. S. Lebedev, "Collision theory of Rydberg atoms with neutral and charged particles". Phys. Rep. 250, 95 (1995).</ref>. The main reasons for such long lifetimes of atoms with excitation energy of several eV are the lack of spatial overlap between the excited circular orbital and low orbitals close to the atom, and the forbidden nature of transitions from high orbitals to low orbitals since the strong selection rule Δ''l'' = -1 is operative in a dipole transition. Similar effects exist in the condensed Rydberg matter: lack of orbital spatial overlap and angular momentum conservation as described make the lifetime of Rydberg matter long. In addition quantum mechanical properties of the system e.g. exchange-correlation effects create an energy barrier (see figure) which further prevents the deexcitation of the valence electrons in the matter since the electrons have to tunnel through the barrier to the low states <ref>E.A. Manykin, M.I. Ojovan, P.P. Poluektov. "Theory of the condensed state in a system of excited atoms". Sov. Phys. JETP 57 (1983) 256-262. </ref>. This means that the valence electrons are distributed extremely non-uniformly in the Rydberg matter causing a significant delay in the decay of excitations compared to non-interacting excited atoms. E.g. the half-life of Rydberg matter made of Cs atoms with a relatively low level of excitation at n = 12 is calculated to be as high as 17 s <ref>É. A. Manykin, M. I. Ozhovan, P. P. Poluéktov, "Decay of a condensate consisting of excited cesium atoms". Zh. Éksp. Teor. Fiz. 102, 1109 (1992) [Sov. Phys. JETP 75, 602 (1992)].</ref>. Both the life-time and also the stability of Rydberg matter against impurity recombination increase rapidly with the quantum-mechanical level of excitation <ref>E.A. Manykin, M.I. Ojovan, P.P. Poluektov. "Impurity recombination of Rydberg matter". JETP 78 (1994) 27-32. </ref>. An extrapolation to n = 80 gives a lifetime close to the presently accepted age of the Universe <ref>L. Holmlid, "Redshifts in space caused by stimulated Raman scattering in cold intergalactic Rydberg Matter with experimental verification". J. Exp. Theor. Phys. JETP 100 (2005) 637-644.</ref>. |
||
The reasons for the long lifetime and high stability of Rydberg matter against ionization are still debated in some parts of the physics community (verbatim), especially in the field studying ultracold plasmas. This is at least partly due to a lack of methods to observe Rydberg matter and Rydberg matter clusters in such experiments. As discussed in the literature <ref>L. Holmlid, "Conditions for forming Rydberg Matter: condensation of Rydberg states in the gas phase versus at surfaces". J. Phys.: Condens. Matter 14 (2002) 13469-13479.</ref>, several experimental problems preventing the formation of Rydberg matter in the typical cold plasma setups have not been solved. Experimental results using the published methods of Rydberg matter formation show lifetimes up to hours in the laboratory <ref>S. Badiei and L. Holmlid, "The Rydberg Matter laser: excitation, delays and mode effects in the laser cavity medium". Appl. Phys. B 81 (2005) 549-559.</ref><ref>R. Svensson and L. Holmlid, "Very low work function surfaces from condensed excited states: Rydberg matter of cesium". Surface Sci. 269/270 (1992) 695-699.</ref>. Since Rydberg matter clusters can be maintained in their excited state by thermal radiation, Rydberg matter can exist for days in the the experiments <ref>L. Holmlid, "Direct observation of circular Rydberg electrons in a Rydberg Matter surface layer by electronic circular dichroism". J. Phys.: Condens. Matter 19 (2007) 276206.</ref><ref>L. Holmlid, "Precision bond lengths for Rydberg Matter clusters K19 in excitation levels n = 4, 5 and 6 from rotational radio-frequency emission spectra". Mol. Phys. 105 (2007) 933-939.</ref>. |
The reasons for the long lifetime and high stability of Rydberg matter against ionization are still debated in some parts of the physics community (verbatim), especially in the field studying ultracold plasmas. This is at least partly due to a lack of methods to observe Rydberg matter and Rydberg matter clusters in such experiments. As discussed in the literature <ref>L. Holmlid, "Conditions for forming Rydberg Matter: condensation of Rydberg states in the gas phase versus at surfaces". J. Phys.: Condens. Matter 14 (2002) 13469-13479.</ref>, several experimental problems preventing the formation of Rydberg matter in the typical cold plasma setups have not been solved. Experimental results using the published methods of Rydberg matter formation show lifetimes up to hours in the laboratory <ref>S. Badiei and L. Holmlid, "The Rydberg Matter laser: excitation, delays and mode effects in the laser cavity medium". Appl. Phys. B 81 (2005) 549-559.</ref><ref>R. Svensson and L. Holmlid, "Very low work function surfaces from condensed excited states: Rydberg matter of cesium". Surface Sci. 269/270 (1992) 695-699.</ref>. Since Rydberg matter clusters can be maintained in their excited state by thermal radiation, Rydberg matter can exist for days in the the experiments <ref>L. Holmlid, "Direct observation of circular Rydberg electrons in a Rydberg Matter surface layer by electronic circular dichroism". J. Phys.: Condens. Matter 19 (2007) 276206.</ref><ref>L. Holmlid, "Precision bond lengths for Rydberg Matter clusters K19 in excitation levels n = 4, 5 and 6 from rotational radio-frequency emission spectra". Mol. Phys. 105 (2007) 933-939.</ref>. |
||
== Numerous excitation levels == |
== Numerous excitation levels == |
||
Revision as of 09:48, 1 May 2009
Definition
Rydberg matter is a solid or liquid state of matter formed from highly excited atoms (see Rydberg atom ) or molecules (see Rydberg molecule ) of the circular Rydberg type [1]. Rydberg matter was predicted around 1980 by E. A. Manykin et al. [2][3]. A circular Rydberg state has its outermost electron in a planar almost circular orbit around the atomic core, like a planet around the Sun. Such Rydberg states are the most long-lived ones, with lifetimes up to several hours in highly excited states in space [4]. Direct studies by laser spectroscopy [5] and emission spectroscopy [6][7] show that Rydberg matter contains circular Rydberg states. Rydberg matter can be formed from many different atoms and molecules. It has been reported to be formed by Cs [8], K [9], H [10], H2 [11] and N2 [12]. It is expected that Rydberg matter can also be formed by other alkali atoms like Na. Observational evidence of Rydberg matter formed by He atoms in space also exists, since the diffuse interstellar bands (DIBs) are well described by doubly excited circular He atomic states embedded in Rydberg matter [13]. Theoretical studies have been made on the formation of Rydberg matter from atoms like Be, Mg and Ca [14].
Physical form
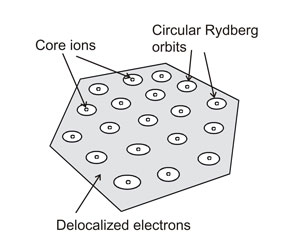
Rydberg matter is a special state of matter, of the same standing as a solid or liquid phase. It is probably a very common form of matter in the Universe [15]. It is not similar to a gas, nor to a plasma, since it forms small particles (clusters) with a crystalline well-defined geometry. The particles are rather small, and large continuous pieces of Rydberg matter cannot exist [16]. In many cases, Rydberg matter is similar to what is called a dusty plasma with small clusters in a gas, like water drops in the air giving a fog or a cloud. Rydberg matter seems to give rise to enormous extended clouds both in space [17][18] and in the upper atmosphere of planets [19]. In the laboratory, the Rydberg matter clouds are easy to study by laser probing [20]. The shape of the small clusters is determined by the circular nature of the Rydberg states from which it is composed, which means that the Rydberg matter clusters normally are planar [21]. Both from theory and experiment it is known that these clusters cannot be very large, and in fact the largest well-defined Rydberg matter cluster observed experimentally so far is composed of only 91 atoms or molecules [22]. The most typical clusters are six-fold symmetric, with all atoms or molecules in one close-packed layer. The planar symmetric shape of the Rydberg matter clusters has been observed directly by rotational spectroscopy [23][24], using their strong stimulated emission signal in the radiofrequency range. This means that the numbers 7, 19, 37, 61 and 91 are the numbers of atoms or molecules giving the most stable six-fold clusters (with complete "circles"). Such numbers are called the magic numbers of a specific type of cluster, in this case of the planar close-packed type. A 19-membered Rydberg matter cluster is shown as example. At the lowest excitation level n = 1, Rydberg matter clusters have a three-dimensional close-packed form [25]. Any attempt to form larger Rydberg matter clusters challenges the retardation effect, which is due to the finite velocity of the electromagnetic interaction (speed of light) [26]. The retardation may affect the strong electron correlation in the Rydberg matter clusters.
Bonding in Rydberg matter

Rydberg matter is formed since the condensed state energetically is more favorable compared with the system of non-interacting circular Rydberg atoms from which it can be considered to be formed [27][28]. (This is not an efficient mechanism of formation in reality). Bonding of excited atoms in the Rydberg matter is due to delocalization of the highly excited valence electrons and therefore its nature is very similar to that of an ordinary metal. In the ordinary description of the number of translational states of a particle in a material like a metal, the electrons move in a small crystal with dimensions a, b and c, forming standing waves reflecting from the edges of the crystal. These standing waves are classified according to quantum numbers describing the number of wavelengths within the distances a, b and c. From this, several properties of a metal can be derived using the fermion nature of the electrons. It is a simple task to make the same type of derivations with the boundary conditions not due to the size of the metal crystal, but instead due to the circular motion of the electrons giving standing waves on closed loops. Thus, the angular momentum is quantized instead of the linear momentum in an ordinary metal. Mathematically, this formulation is very similar to the ordinary description of a metal and is given in standard textbooks [29]. This loop formulation focuses on one important aspect of the metallic properties of Rydberg matter. Thus, Rydberg matter is a generalized type of metal, with a greater variety of properties caused by the widely varying quantum number determining the size of the closed loops. One further important property of Rydberg matter is the highly correlated nature of the electron motion. Without strong electron correlation, no bonding will exist [30]. Thus, the bonding in Rydberg matter is a generalized but also more restricted form of metallic bonding, showing exchange-correlation properties typical for chemical covalent bonding [31]. The electronic excitation and vibrational motion (the phonon distribution) of the bonds in Rydberg matter clusters were studied by Raman and Raman-like spectroscopy [32].
Lifetime

Circular Rydberg states of atoms are extremely long-lived against deexcitation by emission of radiation. The so called radiative lifetime of a circular Rydberg state in n = 100 is approximately 1 second. This means that it decays with a characteristic lifetime of 1 second [33]. The lifetime averaged over the angular momentum quantum numbers is 0.18 s at n = 40 and 17 s for n = 100 [34]. The main reasons for such long lifetimes of atoms with excitation energy of several eV are the lack of spatial overlap between the excited circular orbital and low orbitals close to the atom, and the forbidden nature of transitions from high orbitals to low orbitals since the strong selection rule Δl = -1 is operative in a dipole transition. Similar effects exist in the condensed Rydberg matter: lack of orbital spatial overlap and angular momentum conservation as described make the lifetime of Rydberg matter long. In addition quantum mechanical properties of the system e.g. exchange-correlation effects create an energy barrier (see figure) which further prevents the deexcitation of the valence electrons in the matter since the electrons have to tunnel through the barrier to the low states [35]. This means that the valence electrons are distributed extremely non-uniformly in the Rydberg matter causing a significant delay in the decay of excitations compared to non-interacting excited atoms. E.g. the half-life of Rydberg matter made of Cs atoms with a relatively low level of excitation at n = 12 is calculated to be as high as 17 s [36]. Both the life-time and also the stability of Rydberg matter against impurity recombination increase rapidly with the quantum-mechanical level of excitation [37]. An extrapolation to n = 80 gives a lifetime close to the presently accepted age of the Universe [38].
The reasons for the long lifetime and high stability of Rydberg matter against ionization are still debated in some parts of the physics community (verbatim), especially in the field studying ultracold plasmas. This is at least partly due to a lack of methods to observe Rydberg matter and Rydberg matter clusters in such experiments. As discussed in the literature [39], several experimental problems preventing the formation of Rydberg matter in the typical cold plasma setups have not been solved. Experimental results using the published methods of Rydberg matter formation show lifetimes up to hours in the laboratory [40][41]. Since Rydberg matter clusters can be maintained in their excited state by thermal radiation, Rydberg matter can exist for days in the the experiments [42][43].
Numerous excitation levels
| n | d (nm) | D (cm-3) |
|---|---|---|
| 1 | 0.153 | 2.8×1023 |
| 4 | 2.45 | |
| 5 | 3.84 | |
| 6 | 5.52 | |
| 10 | 15.3 | 2.8×1017 |
| 40 | 245 | |
| 80 | 983 | |
| 100 | 1534 | 2.8×1011 |
Rydberg matter is not just one type of matter like an ordinary metal like sodium (Na), with a quite well defined distance between the atoms. The interatomic distance in sodium metal varies slightly with temperature and pressure, but as long as it is well below the thermodynamic critical point, the interatomic distance both in the solid and liquid phase is almost constant. This is not the case for Rydberg matter, where the interatomic distance varies very rapidly with the electronic state of the material. This means that Rydberg matter is not just one type of matter but many different types with somewhat different properties. The so called principal quantum number n for an atom is also of direct importance in the condensed phase Rydberg matter, but it is named the excitation level n to avoid misunderstandings. This value can vary from n = 1 up to values like n = 100 in space [44][45]. The bond distance (interionic distance) in the different forms of Rydberg matter is given by the formula
where a0 is the Bohr radius equal to 52.9 pm. The approximate factor 2.9 was originally found by theoretical calculations , but it has subsequently been measured with higher precision by rotational spectroscopy in several different types of Rydberg matter clusters [46]. A few typical values of n are given in the table, with bond distances and approximate maximum number densities for the different forms of Rydberg matter. For example Cs atoms with n = 10-15 form Rydberg matter with the number density 1017 - 1018 cm-3. This type of Rydberg matter can be formed in thermionic energy converters (TECs) [47][48]. The electronic spectroscopy of Rydberg matter has been studied in several papers using an intra-cavity (laser cavity) method [49][50]. The electronic levels observed agree with the expected Rydberg matter level structure.
Condensation
Cooled alkali atoms can form Bose-Einstein condensates (BEC). This type of condensate has been studied by atomic physics methods. However, it is not so easy to apply similar methods to form Rydberg matter condensates, due to experimental problems. Several of them were described and discussed in a paper published in 2002 [51], together with a systematic description of the methods successfully used to form Rydberg matter so far. The main problem is naturally that it is impossible to condense a gas in a vacuum without rapidly removing the condensation energy. If no other physical object is used to remove the condensation energy, initial condensation will give rise to ionization and plasma formation and not to fomation of stable Rydberg matter. In all methods used successfully to form Rydberg matter, an adjacent surface removes the excess energy released by the condensation [52]. In fact, the most efficient process is to form the Rydberg matter clusters during desorption (evaporation) from a solid surface, which means that the excess bond energy is deposited in the surface [53]. That the desorption process from non-metal surfaces gives Rydberg species has been verified in numerous papers, with just three examples [54][55][56] provided. Another excellent example of this is the formation of dense Cs Rydberg matter in plasma devices like thermionic energy converters (TECs) [25] with graphite covered electrodes, where the work function of the Rydberg matter covered surfaces can reach values as low as 0.5 eV [57]. Such uniquely low values of the work function indicate directly a very dilute metal, in this case Rydberg matter with n = 10 - 15 [58].
See also
References
- ^ J. Liang, M. Gross, P. Goy, S. Haroche, "Circular Rydberg-state spectroscopy". Phys. Rev. A 33 (1986) 4437-4439
- ^ É. A. Manykin , M. I. Ozhovan, P. P. Poluéktov. "Transition of an excited gas to a metallic state". Sov. Phys. Tech. Phys. Lett. 6 (1980) 95.
- ^ É. A. Manykin, M. I. Ozhovan, P. P. Poluéktov, "On the collective electronic state in a system of strongly excited atoms". Sov. Phys. Dokl. 26 (1981) 974-975.
- ^ R. L. Sorochenko, "Postulation, detection and observations of radio recombination lines (review)" in "Radio recombination lines: 25 years of investigation", eds. M. A. Gordon, R. L. Sorochenko, Kluwer 1990.
- ^ L. Holmlid, "Direct observation of circular Rydberg electrons in a Rydberg Matter surface layer by electronic circular dichroism". J. Phys.: Condens. Matter 19 (2007) 276206.
- ^ L. Holmlid, "Nuclear spin transitions in the kHz range in Rydberg Matter clusters give precise values of the internal magnetic field from orbiting Rydberg electrons". Chem. Phys. 358 (2009) 61–67.
- ^ L. Holmlid, "Stimulated emission spectroscopy of Rydberg Matter: observation of Rydberg orbits in the core ions". Appl. Phys. B 87 (2007) 273-281.
- ^ V. I. Yarygin, V. N. Sidel’nikov, I. I. Kasikov, V. S. Mironov, and S. M. Tulin, "Experimental Study on the Possibility of Formation of a Condensate of Excited States in a Substance (Rydberg Matter)". Pis’ma Zh. Éksp. Teor. Fiz. 77, 330 (2003) [JETP Lett. 77, 280 (2003)].
- ^ S. Badiei and L. Holmlid, "Neutral Rydberg Matter clusters from K: extreme cooling of translational degrees of freedom observed by neutral time-of-flight". Chem. Phys. 282 (2002) 137-146.
- ^ S. Badiei and L. Holmlid, "Experimental studies of fast fragments of H Rydberg matter". J. Phys. B: At. Mol. Opt. Phys. 39 (2006) 4191-4212.
- ^ J. Wang and L. Holmlid, "Rydberg Matter clusters of hydrogen (H2)N* with well defined kinetic energy release observed by neutral time-of-flight". Chem. Phys. 277 (2002) 201-210.
- ^ S. Badiei and L. Holmlid, "Rydberg Matter of K and N2: angular dependence of the time-of-flight for neutral and ionized clusters formed in Coulomb explosions". Int. J. Mass Spectrom. 220 (2002) 127-136.
- ^ L. Holmlid, "The diffuse interstellar band carriers in interstellar space: all intense bands calculated from He doubly excited states embedded in Rydberg Matter". Mon. Not. R. Astron. Soc. 384 (2008) 764-774.
- ^ A. V. Popov, "Search for Rydberg matter: Beryllium, magnesium and calcium". Czechoslovak Journal of Physics 56 (2006) B1294-B1299.
- ^ S. Badiei and L. Holmlid, "Rydberg Matter in space - low density condensed dark matter". Mon. Not. R. Astron. Soc. 333 (2002) 360-364.
- ^ L. Holmlid, "Classical energy calculations with electron correlation of condensed excited states - Rydberg Matter". Chem. Phys. 237 (1998) 11-19.
- ^ L. Holmlid, "Amplification by stimulated emission in Rydberg Matter clusters as the source of intense maser lines in interstellar space". Astrophys. Space Sci. 305 (2006) 91-98.
- ^ L. Holmlid, "The diffuse interstellar band carriers in interstellar space: all intense bands calculated from He doubly excited states embedded in Rydberg Matter". Mon. Not. R. Astron. Soc. 384 (2008) 764-774.
- ^ L. Holmlid, "The alkali metal atmospheres on the Moon and Mercury: explaining the stable exospheres by heavy Rydberg Matter clusters". Planetary Space Sci. 54 (2006) 101-112.
- ^ H. Åkesson, S. Badiei and L. Holmlid, "Angular variation of time-of-flight of neutral clusters released from Rydberg Matter: primary and secondary Coulomb explosion processes". Chem. Phys. 321 (2006) 215-222.
- ^ L. Holmlid, "Classical energy calculations with electron correlation of condensed excited states - Rydberg Matter". Chem. Phys. 237 (1998) 11-19.
- ^ J. Wang and L. Holmlid, "Rydberg Matter clusters of hydrogen (H2)N* with well defined kinetic energy release observed by neutral time-of-flight". Chem. Phys. 277 (2002) 201-210.
- ^ L. Holmlid, "Precision bond lengths for Rydberg Matter clusters K19 in excitation levels n = 4, 5 and 6 from rotational radio-frequency emission spectra". Mol. Phys. 105 (2007) 933-939.
- ^ L. Holmlid, "Rotational spectra of large Rydberg Matter clusters K37, K61 and K91 give trends in K-K bond distances relative to electron orbit radius". J. Mol. Struct. 885 (2008) 122-130.
- ^ L. Holmlid, "Clusters HN+ (N = 4, 6, 12) from condensed atomic hydrogen and deuterium indicating close-packed structures in the desorbed phase at an active catalyst surface". Surf. Sci. 602 (2008) 3381–3387.
- ^ L. Holmlid, "Classical energy calculations with electron correlation of condensed excited states - Rydberg Matter". Chem. Phys. 237 (1998) 11-19.
- ^ É. A. Manykin, M. I. Ozhovan, P. P. Poluéktov, "On the collective electronic state in a system of strongly excited atoms". Sov. Phys. Dokl. 26 (1981) 974-975.
- ^ E.A. Manykin, M.I. Ojovan, P.P. Poluektov. "Rydberg matter: properties and decay". Proc. SPIE 6181 (2006) 618105.
- ^ F. Reif, "Fundamentals of statistical and thermal physics". MacGraw-Hill, New York, 1965.
- ^ L. Holmlid, "Classical energy calculations with electron correlation of condensed excited states - Rydberg Matter". Chem. Phys. 237 (1998) 11-19.
- ^ E.A. Manykin, M.I. Ojovan, P.P. Poluektov. "Theory of the condensed state in a system of excited atoms". Sov. Phys. JETP 57 (1983) 256-262.
- ^ L. Holmlid, "Vibrational transitions in Rydberg Matter clusters from stimulated Raman and Rabi-flopping phase-delay in the infrared". J. Raman Spectr. 39 (2008) 1364-1374.
- ^ J. Liang, M. Gross, P. Goy, S. Haroche, "Circular Rydberg-state spectroscopy". Phys. Rev. A 33 (1986) 4437-4439.
- ^ I. L. Beigman and V. S. Lebedev, "Collision theory of Rydberg atoms with neutral and charged particles". Phys. Rep. 250, 95 (1995).
- ^ E.A. Manykin, M.I. Ojovan, P.P. Poluektov. "Theory of the condensed state in a system of excited atoms". Sov. Phys. JETP 57 (1983) 256-262.
- ^ É. A. Manykin, M. I. Ozhovan, P. P. Poluéktov, "Decay of a condensate consisting of excited cesium atoms". Zh. Éksp. Teor. Fiz. 102, 1109 (1992) [Sov. Phys. JETP 75, 602 (1992)].
- ^ E.A. Manykin, M.I. Ojovan, P.P. Poluektov. "Impurity recombination of Rydberg matter". JETP 78 (1994) 27-32.
- ^ L. Holmlid, "Redshifts in space caused by stimulated Raman scattering in cold intergalactic Rydberg Matter with experimental verification". J. Exp. Theor. Phys. JETP 100 (2005) 637-644.
- ^ L. Holmlid, "Conditions for forming Rydberg Matter: condensation of Rydberg states in the gas phase versus at surfaces". J. Phys.: Condens. Matter 14 (2002) 13469-13479.
- ^ S. Badiei and L. Holmlid, "The Rydberg Matter laser: excitation, delays and mode effects in the laser cavity medium". Appl. Phys. B 81 (2005) 549-559.
- ^ R. Svensson and L. Holmlid, "Very low work function surfaces from condensed excited states: Rydberg matter of cesium". Surface Sci. 269/270 (1992) 695-699.
- ^ L. Holmlid, "Direct observation of circular Rydberg electrons in a Rydberg Matter surface layer by electronic circular dichroism". J. Phys.: Condens. Matter 19 (2007) 276206.
- ^ L. Holmlid, "Precision bond lengths for Rydberg Matter clusters K19 in excitation levels n = 4, 5 and 6 from rotational radio-frequency emission spectra". Mol. Phys. 105 (2007) 933-939.
- ^ L. Holmlid, "Redshifts in space caused by stimulated Raman scattering in cold intergalactic Rydberg Matter with experimental verification". J. Exp. Theor. Phys. JETP 100 (2005) 637-644.
- ^ S. Badiei and L. Holmlid, "Magnetic field in the intracluster medium: Rydberg matter with almost free electrons". Mon. Not. R. Astron. Soc. 335 (2002) L94-L98.
- ^ L. Holmlid, "Rotational spectra of large Rydberg Matter clusters K37, K61 and K91 give trends in K-K bond distances relative to electron orbit radius". J. Mol. Struct. 885 (2008) 122-130.
- ^ V. I. Yarygin, V. N. Sidel’nikov, I. I. Kasikov, V. S. Mironov, and S. M. Tulin, "Experimental Study on the Possibility of Formation of a Condensate of Excited States in a Substance (Rydberg Matter)". Pis’ma Zh. Éksp. Teor. Fiz. 77, 330 (2003) [JETP Lett. 77, 280 (2003)].
- ^ R. Svensson, L. Holmlid and L. Lundgren, "Semi-conducting low pressure, low temperature plasma of cesium with unidirectional conduction". J. Appl. Phys. 70 (1991) 1489-1492.
- ^ L. Holmlid, "Stimulated emission spectroscopy of Rydberg Matter: observation of Rydberg orbits in the core ions". Appl. Phys. B 87 (2007) 273-281.
- ^ S. Badiei and L. Holmlid, "The Rydberg Matter laser: excitation, delays and mode effects in the laser cavity medium". Appl. Phys. B 81 (2005) 549-559.
- ^ L. Holmlid, "Conditions for forming Rydberg Matter: condensation of Rydberg states in the gas phase versus at surfaces". J. Phys.: Condens. Matter 14 (2002) 13469-13479.
- ^ L. Holmlid, "Conditions for forming Rydberg Matter: condensation of Rydberg states in the gas phase versus at surfaces". J. Phys.: Condens. Matter 14 (2002) 13469-13479.
- ^ J. Wang, K. Engvall and L. Holmlid, "Cluster KN formation by Rydberg collision complex stabilization during scattering of a K beam off zirconia surfaces". J. Chem. Phys. 110 (1999) 1212-1220.
- ^ L. Holmlid, "Complex kinetics of desorption and diffusion. Field reversal study of K excited-state desorption from graphite layer surfaces". J. Phys. Chem. A 102 (1998) 10636-10646.
- ^ Kotarba, A., Baranski, A., Hodorowicz, S., Sokolowski, J., Szytula, A., Holmlid L. "Stability and excitation of potassium promoter in iron catalysts - the role of KFeO2 and KAlO2 phases". Catal. Lett. 67 (2000) 129-134.
- ^ Kotarba, A., Adamski, G., Sojka, Z., Witkowski, S., Djega-Mariadassou, G. "Potassium at catalytic surfaces - stability, electronic promotion and excitation". Studies in Surface Science and Catalysis 130A (2000) 485-490.
- ^ R. Svensson and L. Holmlid, "Very low work function surfaces from condensed excited states: Rydberg matter of cesium". Surface Sci. 269/270 (1992) 695-699.
- ^ É. A. Manykin, M. I. Ozhovan, P. P. Poluéktov, "Decay of a condensate consisting of excited cesium atoms". Zh. Éksp. Teor. Fiz. 102, 1109 (1992) [Sov. Phys. JETP 75, 602 (1992)].

