Phosphinimide ligands: Difference between revisions
Gregmcginnis (talk | contribs) |
Gregmcginnis (talk | contribs) |
||
| Line 22: | Line 22: | ||
In most respects the sterics of phosphinimide ligands and electronic properties are considered analogous to cyclopentadienyl ligands(7,8,9). Stericly the metal bound phosphinimide ligand t-Bu3PN produces a cone angles of 87°, quite similar to 83° for cylclopentadienyl suggesting they occupy similar area(8). However, this does not take into account that the bulkyness of the phosphinimide ligand is partly removed from the metal, by the distance of the nitrogen metal bond. This removal of bulkyness can be considered a beneficial to catalysis as one of the strategies to increase polymerization is to increase the exposure of the metal centre(8). The consequence to a less stericly crowded metal centre appears to be increased deactivation of the catalysis(7,8,9). |
In most respects the sterics of phosphinimide ligands and electronic properties are considered analogous to cyclopentadienyl ligands(7,8,9). Stericly the metal bound phosphinimide ligand t-Bu3PN produces a cone angles of 87°, quite similar to 83° for cylclopentadienyl suggesting they occupy similar area(8). However, this does not take into account that the bulkyness of the phosphinimide ligand is partly removed from the metal, by the distance of the nitrogen metal bond. This removal of bulkyness can be considered a beneficial to catalysis as one of the strategies to increase polymerization is to increase the exposure of the metal centre(8). The consequence to a less stericly crowded metal centre appears to be increased deactivation of the catalysis(7,8,9). |
||
[[File:Cone Angles of Phosphinimide Ligands and Cyclopentadienyl Ligands.png| |
[[File:Cone Angles of Phosphinimide Ligands and Cyclopentadienyl Ligands.png|right|Cone angles of a tert-butyl phosphinimide ligands and cyclopentadienyl ligands when bonded to a metal centre.]] |
||
In order to determine the efficiency of each ligand on catalysis experiments must be performed to determine the rate of reactions and quality of product. Experimentally, ethylene catalysis done by phosphinimide ligands complexes showed similar reactivity when compaired to cyclopentadienyl lingands. A slightly lower reaction rate and PE Molecular Weight where obtained using phosphinimide ligands however, a lower pdi was observed(9). It should be noted that in terms of ethylene polymerization phosphinimide ligand catalysis are new when compared to cyclopentadienyl complexes and therefore further development is likely to increase this catalytic rate. |
In order to determine the efficiency of each ligand on catalysis experiments must be performed to determine the rate of reactions and quality of product. Experimentally, ethylene catalysis done by phosphinimide ligands complexes showed similar reactivity when compaired to cyclopentadienyl lingands. A slightly lower reaction rate and PE Molecular Weight where obtained using phosphinimide ligands however, a lower pdi was observed(9). It should be noted that in terms of ethylene polymerization phosphinimide ligand catalysis are new when compared to cyclopentadienyl complexes and therefore further development is likely to increase this catalytic rate. |
||
Revision as of 05:15, 19 November 2012
Structure and Bonding
Phosphinimide ligands have the general formula NPR3-, where the R groups represent organic substituents or, in rare cases, halides or NR2 groups.(4) A discrete ion of NPR3- is not known, but the ligand can be easily derived from neutral phosphinimine precursors.(4,5)

Several coordination types have been observed for transition metal phosphinimide complexes including terminal, μ2-N-bridging, and μ3-N-bridging.(4) In the terminal bonding mode the ligand can have either a linear or a bent geometry at Nitrogen, and in the μ2-N-bridging mode four-membered M2N2 rings can be formed either in an unsymmetrical fashion or in a symmetrical fashion. (4,1) The preferred coordination type is largely dependent upon the oxidation state of the metal as well as, to a lesser extent, upon the ligand sphere of the metal and the steric and electronic properties of the R groups on phosphorous.(4) Bonding modes (A) and (B) are primarily seen in complexes containing metals in high oxidation states, mode (E) is preferred by metals with low oxidation states, and modes (C) and (D) are preferred by metals with medium oxidation states.(4)
Properties and Reactivity
The phosphinimide ligand is isoelectronic with ligands such as [OSiMe3]-, OPR3 and NSiR3.(4) The R groups on P can be varied to give a variety of ligands with different electronic and steric properties. Due to the high oxidation state of phosphorous, phosphinimide ligands have good thermal stability.[1]
Synthesis
(6,1)
Applications
Phosphinimide Polyethylene Catalysts
History
Phosphinimide ligands have shown recent promise in the area of ethylene polymerization. Since 1955 this field has been dominated by metallocene based catalysts starting with the Ziegler catalyst, followed shortly by the kaminsky catalyst in 1976(10,11). The initial design bases for phosphinimide ligands was suggested due to the fact they have similar steric and electronic properties to metallocene catalysts(8).
Phosphinimide Ligands Compared to Cylclopentadienyl Ligands in Ethylene Polymerization
In most respects the sterics of phosphinimide ligands and electronic properties are considered analogous to cyclopentadienyl ligands(7,8,9). Stericly the metal bound phosphinimide ligand t-Bu3PN produces a cone angles of 87°, quite similar to 83° for cylclopentadienyl suggesting they occupy similar area(8). However, this does not take into account that the bulkyness of the phosphinimide ligand is partly removed from the metal, by the distance of the nitrogen metal bond. This removal of bulkyness can be considered a beneficial to catalysis as one of the strategies to increase polymerization is to increase the exposure of the metal centre(8). The consequence to a less stericly crowded metal centre appears to be increased deactivation of the catalysis(7,8,9).
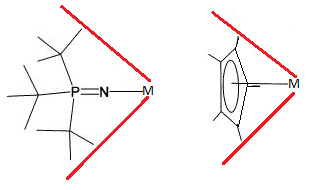
In order to determine the efficiency of each ligand on catalysis experiments must be performed to determine the rate of reactions and quality of product. Experimentally, ethylene catalysis done by phosphinimide ligands complexes showed similar reactivity when compaired to cyclopentadienyl lingands. A slightly lower reaction rate and PE Molecular Weight where obtained using phosphinimide ligands however, a lower pdi was observed(9). It should be noted that in terms of ethylene polymerization phosphinimide ligand catalysis are new when compared to cyclopentadienyl complexes and therefore further development is likely to increase this catalytic rate.
The phosphinimide ligands provide an alternative to metallocene catalysts due to their capability to be easily altered during synthesis, allowing for immense variation in the sterics and electronics properties of the system. It should be noted that steric modifications of phosphinimide ligands have shown to affect the rate of catalysis much more than electronic modifications(8). Therefore, increased specificity of the catalytic properties may be obtained. Also mechanistic and catalytic studies of PE synthesis may be obtained easier through substituent variation and 31P nmr analysis.
General Catalysis Scheme
Activation
The catalyst must be activated in order for polymerization to occur. MAO is a common cocatalyst (activator) for PE polymerization. The discrete structure of MAO is unknown as is the mechanism of this is reaction however, a general activation scheme has been purposed(10,11,12).
Scheme1 scheme 2
Another scheme uses a strong organo-lewis acids such as B(C6F5)3 as a cocatalyst to activate the polymerization through methyl abstraction(9,11) producing a highly reactive zwitterion form(9). Different methods of activation have shown to produce different ethylene polymerization activity(8,9) making it a very important concept to analyse. In general it is observed that phosphinimide catalysts show less activity when activated with MAO(9).
Mechanism of Polymerization
The catalyst is thought to be a homogenous, single sited and therefore produces pdi comparable to metallocene catalysts(8,9) which are also believed to be homogenous, single sited catalysts. The catalytic process is assumed to proceed in much of the same way as metallocene based catalysts as ethylene is thought to interact primarily with the metal centre and not through the bulky ligands(7,12).
Mechanism
Deactivation
Catalytic degredation most commonly occurs from cationic dimerization, lewis acid interactions (often with AlMe3 cocatalyst (MAO)) activating a C-H bond or by excess boron (from another cocatalysts B(C6F5)3) reacting with the active zwitterion(8,9). Catalytic degradation has been shown to occur slower with bulkier phosphinimide ligands(8,9) which prevent such interactions.
-Activation 11 -olefin polymerization (1,3,7,8 -industrial 9 -general catalysis(2,10)
References
1.http://pubs.acs.org/doi/full/10.1021/om050096b
2.http://search.proquest.com/docview/305323031
3.http://www.nrcresearchpress.com/doi/pdf/10.1139/v06-071
4.http://www.sciencedirect.com/science/article/pii/S001085459800191X
5.http://www.sciencedirect.com/science/article/pii/S0065305505540061
6.http://www.sciencedirect.com/science/article/pii/S0010854597900552#
7.http://pubs.acs.org.ezproxy.library.uvic.ca/doi/pdf/10.1021/om060565p
8.http://pubs.acs.org.ezproxy.library.uvic.ca/doi/pdf/10.1021/om020954t
11.http://search.proquest.com/docview/304631081/abstract?accountid=14846
12.http://www.huntresearchgroup.org.uk/teaching/teaching_comp_chem_year4/Case_3_TiCat.pdf
James Funk Amanda Charpentier Greg McGinnis
